
Neuroprotective Effects of Korean Ginseng Berry against Hydrogen Peroxide-induced Oxidative Damage in SH-SY5Y Neuroblastoma Cells
#Seul A Jin and Su Kang Kim are contributed equally to this paper
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Studies on the efficacy of ginseng have focused on the ginseng root. Recently, research on ginseng berries has expanded, revealing its efficacy in liver disease, obesity, and diabetes. This study aimed to examine the neuroprotective effects of Korean ginseng berry (KGB) extract on hydrogen peroxide (H2O2)-induced oxidative stress in human neuroblastoma SH-SY5Y cells
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, reactive oxygen species (ROS) generation, mitochondrial membrane potential, and protein expression analysis were used to evaluate the cytotoxicity and inhibition of apoptosis in SH-SY5Y cells. KGB pretreatment reduced ROS production and mitochondrial dysfunction induced by H2O2-mediated apoptosis in human neuroblastoma SH-SY5Y cells, while also inhibiting Bax upregulation, Bcl-2 downregulation, and caspase-3 activation. Moreover, KGB treatment increased the activity of antioxidant enzymes including superoxide dismutase, glutathione peroxidase, and catalase and decreased the malondialdehyde content.
This study found that KGB protected human neuroblastoma SH-SY5Y cells from H2O2-induced oxidative stress by inhibiting the MAPK signaling pathway. Based on these findings, KGB may be a potential therapeutic agent for preventing and treating neurodegenerative diseases and other disorders associated with oxidative stress.
Keywords:
Human Neuroblastoma, Korean Ginseng Berry, Neurodegenerative Diseases, Neuroprotection, Oxidatve DamageINTRODUCTION
Alzheimer's disease and Parkinson's disease are neurode-generative disorders that are characterized by the death of neurons in the brain. Oxidative stress in degenerative diseases is known as a major cause. Decreased antioxidants, mitochondrial abnormalities, and increased reactive oxygen species (ROS)-related cell death due to oxidative stress have been reported in neurodegenerative diseases (Singh et al., 2019).
Organisms produce ROS with strong oxidizing power such as superoxide anion (O2·-), hydrogen peroxide (H2O2), and the hydroxyl radical (OH·) (He et al., 2017). Oxygen free radical refers to oxygen that is in an unstable state, unlike the oxygen that we normally breathe (Monteiro e Silva et al., 2017). A proper of ROS is necessary for cells to grow and protect the body from pathogens with a strong bactericidal action, but excessive ROS could oxidize various compounds in the human body.
Oxidation causes oxidative stress by damaging or transforming important components such as lipids, proteins and DNA (Singh et al., 2019). This oxidative stress induces cell death of neurons in the brain (Brieger et al., 2012). When ROS is generated excessively and oxidative stress is continuously accumulated in the body, it could affect and damage the gene of the cell and exacerbates the immune system.
Therefore, inhibition of ROS generation may be helpful in preventing and treating neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and Huntington's disease. A lot of research is being conducted to develop effective and safe antioxidants that can be usefully used for the treatment or prevention of neuro-degenerative diseases.
The use of ginseng as herbal medicine is also attracting attention in modern medicine. Research on the chemical composition and physiological effects of ginseng continues, and it is known that ginsenosides, a chemical component of ginseng, can exhibit various physiological effects.
Ginseng has various physiological effects such as antioxidant, anti-cancer, anti-inflammatory, anti-stress, and immune regulation, and is applied to cancer prevention and treatment, immunity enhancement, and fatigue recovery (Tan et al., 2015; Yuan et al., 2016).
The ginseng berry is red berries which can only be harvested in 4 years after ginseng was cultivated. Until recently, the efficacy of ginseng has been mainly concentrated on the ginseng root, and the ginseng berry was considered useless.
However, accumulated evidence suggests that ginseng berry has more pharmacological activities than the root (Dey et al., 2003; Kim et al., 2012; Lee et al., 2014; Cho et al., 2018). Moreover, recent studies have demonstrated that ginseng berries were evaluated to have higher Re, Rb2, Rc, Rd, Rg1, and Rg2 contents than ginseng roots. (Shao et al., 2004; Kim et al., 2012; Cho et al., 2013a; Jung et al., 2016).
In particular, ginseng berry extract contains a high level of ginsenoside Re among ginsenosides, which is almost 30 - 40 times that of ginseng root, and this result has been confirmed in previous studies. (Yi et al., 2018; Byun et al., 2021). It has been reported that ginseng berry has antioxidant (Shao et al., 2004), immunostimulatory (Zhang et al., 2015), anti-cancer (Jung et al., 2016), and anti-hyperlipidemia effect (Mehendale et al., 2006; Cho et al., 2013b). It contains as much ginsenoside as ginseng root and has efficacy against various diseases, but not much research has been done on degenerative brain diseases.
In the present study, the protective effect of Korean ginseng berry extract (KGB) against H2O2 induced oxidative stress was investigated in human neuroblastoma SH-SY5Y cells. So, we evaluated the possibility of neuroprotection against degenerative brain diseases through whether it is effective in suppressing apoptosis and oxidative stress.
MATERIALS AND METHODS
1. Preparation of Korean ginseng berry extract (KGB)
The extract of KGB was provided from food ingredient & product company (ShinWoo, Anyang, Korea). The berry containing seeds of Korean ginseng (Panax ginseng C. A. Meyer) were harvested in 2019, and the cultivation period was 4 years. The KGB was processed from the fruit excluding seeds to extract.
The KGB was placed in 80% alcohol for 8 h. After removing alcohol, the KGB was extracted in extractor with purified water at 80℃ for 8 h. The extract was filtered with 12 ㎛ filter paper (Hyundai Micro Co., Ltd., Seongnam, Korea). The filtrate was concentrated under reduced pressure to have a solid content of more than 65%. The final yield was 2%. The extracted KGB contains 65 ㎎/g of Re component and 16 ㎎/g of Rg1 + Rb1 + Rg3 component.
2. Cell culture and treatments
The human neuroblastoma SH-SY5Y cell line was purchased from American Type Culture Collection (Manassas, VA, USA) and was maintained in a 1 : 1 mixture of Dulbecco’s Modified Eagle Medium and Nutrient F-12 medium (DMEM/F-12) (Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum and 1% (v/v) penicillin (100 U/㎖)/streptomycin (100 ㎍/㎖) at 37℃ in a humidified atmosphere containing 5% CO2.
KGB extracts were dissolved in culture medium before use. To evaluate the inhibition of induction of apoptosis, the whole KGB extract was treated in advance for 1 h, and cell death was induced by exposing the cells to H2O2 at a concentration of 100 μM for 24 h.
3. Cell viability assay
The cell viability of SH-SY5Y cells cultured under various conditions was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
To this end, after the KGB extract treatment at an appropriate concentration was completed, the medium was removed, and formazan formed in each well was dissolved in DMSO by treating with 0.5 ㎎/㎖ MTT (Sigma-Aldrich Co., St Louis, MO, USA) solution at 37℃ in the dark. After 3 h, 200 ㎕ of the reaction solution was transferred to a 96 well plate, and the change in absorbance at 540 ㎚ was measured using an Enzyme-Linked ImmunoSorbent Assay (ELISA) reader (Thermo Fisher Scientific Inc., Willington, CT, USA).
4. Hoechst 33342 staining
To perform Hoechst 33342 staining, the cell medium was removed and washed three times with 1 × phosphate beffered saline (PBS, Mediatech Inc., Manassas, VA, USA). For fixation after washing, fixation was performed in 4% paraformaldehyde at room temperature for 30 min. And it was dyed using a staining reagent (Hoechst 33342) 1 ㎍/㎖ in PBS for 20 min in a dark room.
Apoptosis and DNA damage were observed under a fluorescence microscope (Nikon Instruments Inc., Tokyo, Japan). Data were shown as a percentage of apoptotic cells with respect to the total cell number.
5. Detection of ROS generation and mitochondrial membrane potential (MMP)
To evaluate intracellular ROS production, a fluorescent probe known as 2',7'-dichlorofluorescein diacetate (DCFH-DA) was utilized. The SH-SY5Y cell were seeded in black 96-well plates and allowed to adhere for 24 hours. The cells were washed three with PBS, and then loaded with DCFH-DA 20 μM (Sigma-Aldrich Co., St Louis, MO, USA) and incubated at 37℃ for 30 min. The fluorescence was measured at an excitation wavelength of 480 ㎚ and an emission wavelength of 520 ㎚ using a fluorescence microplate reader (Molecular Devices LLC, San Jose, CA, USA).
To assess MMP, the fluorescent dye Rhodamine 123 (Rho 123, (Sigma-Aldrich Co., St Louis, MO, USA) was used. The cells were incubated with Rho 123 (10 ㎍/㎖) for 30 min at 37℃ in the dark. The fluorescence image was captured using a fluorescence microscope at an emission wavelength of 529 ㎚.
6. Western blot analysis
After treatment with KGB extract and H2O2, proteins were extracted from cells after 24 h.
The radioimmunoprecipitation assay (RIPA) buffer was used to extract proteins. RIPA buffer was added to the cells collected by centrifugation, mixed well, and centrifuged at 13,000 rpm for 20 min. After centrifugation, the supernatant was transferred to a new tube and the amount of protein in the extracted protein was measured by the Bradford method. Before Western blotting, the amount of protein in each sample was equally quantified as 20 ㎍.
The quantified proteins were subjected to electrophoresis on SDS-PAGE with loading dye for western blotting. Electrophoresis was performed at 80V for 2 h. For SDS-PAGE electrophoresis, a nitrocellulose membrane (Life sciences, Pocheon, Korea) was used to transfer proteins to membrane. Transfer conditions were performed at 250 ㎷ for 3 h.
To evaluate the expression of each specific protein, the membrane transferred protein was incubated in 5% skim milk for 1 h. Each primary antibody (Cell Signaling Technology Inc., Danvers, MA, USA) was then added to the membrane after a 1 : 1000 dilution and incubated overnight at 4℃. After washing with TBS wash buffer, the membrane was incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology Inc., Danvers, MA, USA) diluted 1 : 2000.
Bands corresponding to the target proteins were developed by Chemi-Doc (Thermo Fisher Scientific Inc., Willington, CT, USA) using a chemiluminescent reaction (Santa Cruz Biotechnology Inc., Dallas, Texas, USA). β-actin was used as an internal control.
7. Caspase-3 activity and measurement of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) activities and malondialdehyde (MDA) level
In brief, cell lysis and subsequent centrifugation were conducted to obtain the supernatant. The obtained supernatant was then mixed with the reaction buffer (including DTT) and caspase-3 substrate (Ac-DEVD-pNA) (R&D Systems Inc., Minneapolis, MN, USA) to evaluate caspase-3 activity.
Additionally, assays were carried out to determine the levels of SOD, CAT, GSH-Px activities, and MDA levels (Cayman Chemical, Ann Arbor, Michigan, USA). The absorbance of the reactions was measured using a microplate reader following the manufacturer's protocols.
8. Statistical analysis
Statistical differences between groups were analyzed using the SPSS 26.0 software program (IBM Co., Armonk, NY, USA).
The results of each experiment were expressed as mean and standard error of the mean (S.E.M.) values of the data obtained from three independent experiments performed in two or three wells. Significance was confirmed by the Tukey’s test after one way analysis of variance (ANOVA). After statistical processing, if the p value was less than 0.05, it was considered as significant difference between groups.
RESULTS
1. Effects of Korean ginseng berry extract (KGB) on H2O2-treated cytotoxicity in SH-SY5Y cells
To assess effects of KGB on H2O2-induced cell death, the MTT assay was performed. When KGB was treated with concentrations of 10, 50, 100, and 500 ㎍/㎖, it was found that there was no toxicity to the cells (Fig. 1A).
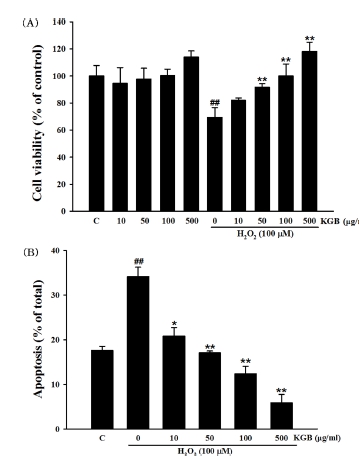
Cell viability evaluation in SH-SY5Y cells treated with H2O2.(A) Cell viability evaluation of Korean ginseng berry extract on H2O2-induced cell death in SH-SY5Y cells using MTT. (B) Results of apoptotic cells by Hoechst 33342 staining. Values were shown as means ± standard error (S.E.M.) by the Tukey’s test after One-way analysis of variance (ANOVA). Symbol indicates a statistically significant value compared to the control (##p < 0.01). *p < 0.05 and **p < 0.01 compared with H2O2 treated cells. C; control, KGB; Korean ginseng berry extract.
In a previous study, we confirmed that when the cells were exposed with various concentrations of H2O2 (10 μM - 200 μM) for 24 h, cell viability was decreased in a dose-dependent manner (Kim et al., 2015). In this study, 100 μM H2O2 was used to assess effect of KGB on H2O2-induced cell death based on previous experimental result. The cell viability of cells exposed to 100 μM H2O2 was 69.35 ± 7.22% of control value.
Pretreatment of KGB significantly showed inhibitory effects on H2O2-induced decrease of cell viability in dose-dependent manners. The viability of SH-SY5Y cells was 82.00 ± 1.70%, 91.61 ± 2.73%, 100.00 ± 8.87%, and 118.16 ± 6.83% at concentration of 10, 50, 100, and 500 ㎍/㎖ of KGB, respectively.
To evaluate the potential inhibitory effects of KGB on H2O2-induced apoptotic cell death in SH-SY5Y cells, an additional experiment was performed utilizing Hoechst 33342 staining. In cells treated with H2O2, DNA condensation and nuclear fragmentation were observed, while control cells displayed round blue nuclei characteristic of viable cells.
However, pretreatment with KGB inhibited these apoptotic characteristics. The proportion of apoptotic cells was calculated and presented in Fig. 1B. The rate of apoptosis in cells treated with H2O2 was 34.09 ± 2.15%, whereas untreated cells exhibited a lower rate of 17.57 ± 0.95%. Nevertheless, treatment with KGB at doses of 10, 50, 100, and 500 ㎍/㎖ resulted in significant dose-dependent reductions in H2O2-induced apoptotic cell death, with values of 20.79 ± 1.93%, 17.06 ± 0.4%, 12.37 ± 1.65%, and 5.85 ± 1.91%, respectively.
2. Inhibitory effects of Korean ginseng berry extract (KGB) on H2O2-induced ROS production
To measure the levels of intracellular ROS, the fluorescent dye DCFH-DA was used, which is oxidized to fluorescent DCF by ROS.
As shown in Fig. 2, the ROS production was increased to up of 260.13 ± 6.50% of control in the cells treated with 100 μM H2O2. However, pretreatment with KGB (10, 50, 100, and 500 ㎍/㎖) significantly reduced the ROS production to 199.73 ± 8.30%, 185.48 ± 6.18%, 157.96 ± 6.48%, and 113.76 ± 4.22%, respectively.
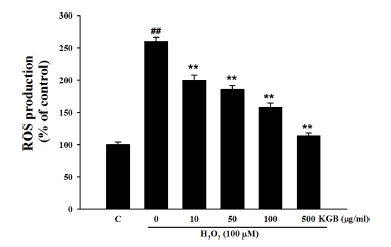
Effects of Korean ginseng berry extract (KGB) on the H2O2-induced ROS generation.Values were shown as mean ± S.E.M. by the Tukey’s test after One-way analysis of variance (ANOVA). Symbol indicates a statistically significant value compared to the control (##p < 0.01). *p < 0.05 and **p < 0.01 compared with H2O2 treated cells. C; control, KGB; Korean ginseng berry extract, ROS; reactive oxygen species.
3. Effects of Korean ginseng berry extract (KGB) on H2O2-induced mitochondrial membrane potential (MMP) loss
To measure mitochondrial membrane potential (MMP), the the fluorescent Rhodamine 123 dye was utilized. Decreased fluorescence intensity of Rho123 indicates mitochondrial membrane depolarization.
In Fig. 3, when SH-SY5 cells were exposed to 100 μM H2O2, the Rho123 fluorescence intensity was significantly reduced to 72.99 ± 3.23% of the control group. However, pretreatment with KGB at concentrations of 10, 50, 100, and 500 ㎍/㎖ increased the fluorescence intensity of Rho123 to 87.68 ± 1.21%, 93.71 ± 2.63%, 98.64 ± 3.00%, and 106.45 ± 3.29%, respectively. These findings suggest that KGB can protect against H2O2-induced mitochondrial damage.
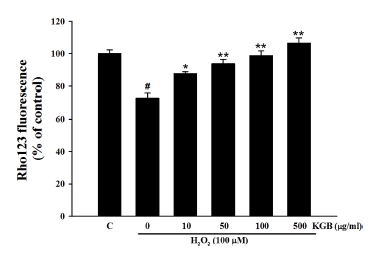
Effects of Korean ginseng berry extract (KGB) on the H2O2-induced MMP loss.Rhodamine 123 (Rho 123), a fluorescent dye, was used for the quantitative expression of MMP. Values are presented as mean ± S.E.M. by the Tukey’s test after One-way analysis of variance (ANOVA). Symbol indicates a statistically significant value compared to the control (#p < 0.05). *p < 0.05 and **p < 0.01 compared with H2O2 treated cells. MMP; mitochondrial membrane potential, C; control, KGB; Korean ginseng berry extract.
4. Effects of Korean ginseng berry extract (KGB) on the expression of Bcl-2 family
The western blot analysis was conducted to explore the potentialmechanisms of KGB on cell injury and assessed the expression of Bcl-2 family members, including Bcl-2 and Bax.
The results indicated that exposure to 100 μM H2O2 decreased the ratio of Bcl-2/Bax to 84.56 ± 0.89% of the control group. However, treatment with KGB at concentrations of 10, 50, 100, and 500 ㎍/㎖ rescued the decreasing ratio, resulting in values of 130.08 ± 0.79%, 153.98 ± 2.43%, 161.70 ± 3.18%, and 168.36 ± 3.63%, respectively, as shown in Fig. 4.
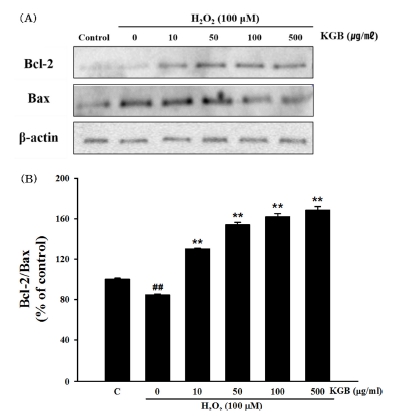
Effects of Korean ginseng berry extract (KGB) on the expression of Bcl-2 family proteins.(A) The expressions of Bcl-2 and Bax proteins. (B) The quantitative analysis of Bcl-2/Bax ratio. The data were showed as a ratio compared to control cells. Values are shown as mean ± S.E.M. by the Tukey’s test after One-way analysis of variance (ANOVA). Symbol indicates a statistically significant value compared to the control (##p < 0.01). **p < 0.01 compared with H2O2-treated cells. C; control, KGB; Korean ginseng berry extract.
5. Effects of Korean ginseng berry extract (KGB) on the expression and activation of caspase-3
The results presented in Fig. 5A and 5B indicate that treatment with H2O2 (100 μM) significantly increased caspase-3 expression by 149.79 ± 6.22% compared to the control group, whereas pretreatment with KGB (at 10, 50, 100, and 500 ㎍/㎖) dose-dependently reduced caspase-3 expression to 144.97 ± 6.26%, 120.71 ± 4.64%, 101.46 ± 3.30%, and 73.73 ± 2.43%, respectively.
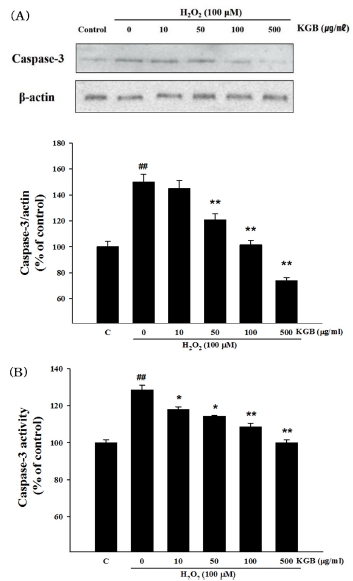
Effects of Korean ginseng berry extract (KGB) on the expression and activation of caspase-3.(A) The expression of caspase-3 protein and â-actin. (B) The activity of caspase-3. The data was showed as a ratio compared to control cells. Values are shown as mean ± S.E.M. by the Tukey’s test after One-way analysis of variance (ANOVA). Symbol indicates a statistically significant value compared to the control (##p < 0.01). *p < 0.05 and **p < 0.01 compared with H2O2 treated cells. C; control, KGB; Korean ginseng berry extract.
Additionally, the alteration of caspase-3 activity in SH-SY5Y cells treated with H2O2 plus KGB was shown in Fig. 5C. Exposure to 100 μM H2O2 significantly increased caspase-3 activity to 128.54 ± 2.44% compared to the control group, while pretreatment with KGB (at 10, 50, 100, and 500 ㎍/㎖) dose-dependently inhibited caspase-3 activity to 117.86 ± 1.51%, 114.34 ± 0.39%, 108.54 ± 2.00%, and 99.98 ± 1.59%, respectively.
6. Effects of Korean ginseng berry extract (KGB) on the activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and the malondialdehyde (MDA) level
The exposure of cells to 100 μM H2O2 reduced the activities of SOD, CAT, and GSH-Px to 39.1 ± 2.64%, 60.45 ± 3.42%, and 64.77 ± 0.92% of the control group, respectively (Fig. 6A - Fig. 6C). However, pretreatment with KGB (10, 50, 100, and 500 ㎍/㎖) significantly increased the activity of SOD (44.00 ± 3.77%, 52.71 ± 2.36%, 71.50 ± 3.14%, and 76.84 ± 2.06%, respectively), CAT (80.25 ± 7.91%, 84.17 ± 6.70%, 107.15 ± 4.47%, and 117.29 ± 4.35%, respectively), and GSH-Px (85.25 ± 7.14, 92.63 ± 7.77, 103.43 ± 7.35, and 128.32 ± 7.97%, respectively).
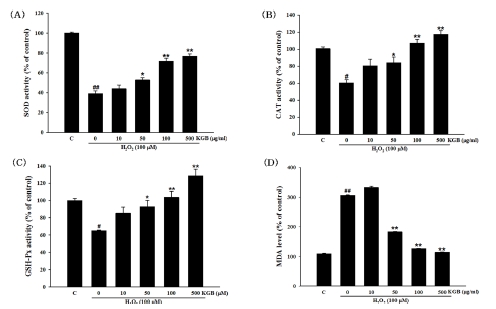
The activity of SOD, CAT, and GSH-Px, and the MDA level.(A) The SOD activity. (B) The CAT activity. (C) The GSH-Px activity. (D) The MDA level. Values were presented as mean ± S.E.M. by the Tukey’s test after One-way analysis of variance (ANOVA). Symbol indicates a statistically significant value compared to the control (#p < 0.05 and ##p < 0.01). *p < 0.05 and **p < 0.01 compared with H2O2 treated cells. SOD; superoxide dismutase, CAT; catalase, GSH-Px; glutathione peroxidase, MDA; malondialdehyde, C; control, KGB; Korean ginseng berry extract.
Further, after the exposure of cells to 100 μM H2O2, the MDA level was markedly increased to 305.56 ± 1.72% of the control, whereas KGB reduced the MDA production to 183.35 ± 1.41%, 126.25 ± 1.17%, and 114.45 ± 1.11% at the concentration of 50, 100, and 500 ㎍/㎖, respectively (Fig. 6D).
7. Effects of Korean ginseng berry extract (KGB) on phosphorylation of mitogen-activated protein kinases (MAPKs)
Western blotting was performed to examine the effect of KGB on p38, JNK, and ERK1/2 protein phosphorylation in the MAPK pathway in response to hydrogen peroxide-induced oxidative stress. As shown in Fig. 7, treatment with H2O2 significantly increased the expression of phosphorylated p38, JNK, and ERK1/2 proteins when compared to the control group. Conversely, pretreatment with KGB effectively decreased the levels of phosphorylated MAPKs in comparison to the H2O2-treated group.
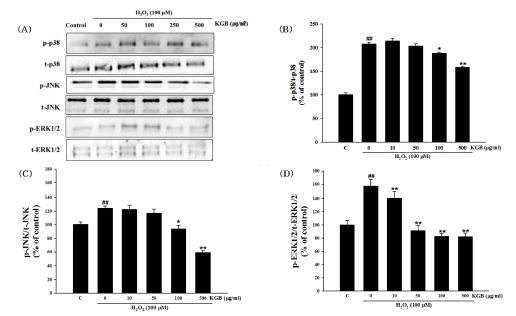
Effects of Korean ginseng berry extract (KGB) on the phosphorylation of mitogen-activated protein kinases (MAPKs).(A) The phosphorylation of MAPKs, including p38, JNK, and ERK1/2 was investigated by Western blot analysis. (B and D) Expression of total form of each protein and relative expression of phosphorylated proteins in each group. Values are shown as mean ± S.E.M. by the Tukey’s test after One-way analysis of variance (ANOVA). Symbol indicates a statistically significant value compared to the control (##p < 0.01). *p < 0.05 and **p < 0.01 compared with H2O2 treated cells. C; control, KGB; Korean ginseng berry extract.
DISCUSSION
Oxidative stress and free radical production may affect cell proliferation, differentiation, cell death, and survival (Hu et al., 2015; Park et al., 2015). Oxidative stress is a state of imbalance between the production of ROS and the body's ability to neutralize them or repair the damage caused by them. ROS include free radicals such as superoxide anion (O2-) and hydroxyl radical (OH-), and non-radicals like H2O2. ROS can cause damage to important cellular components like proteins, lipids, and DNA, leading to cellular dysfunction and even cell death (Tian et al., 2015). Neurodegenerative diseases are known to be associated with high levels of oxidative stress, leading to neurotoxicity and neuronal cell death.
The purpose of this study was to examine the impact of KGB on oxidative stress and cell death induced by H2O2 in human neuroblastoma SH-SY5Y cells. The findings of the study indicated that KGB was able to mitigate mitochondrial dysfunction and intracellular oxidative stress. Consequently, it reduced apoptotic cell death triggered by H2O2 in human neuroblastoma SH-SY5Y cells.
Regulation of biochemical changes is a critical function of oxidative stress (Uttara et al., 2009), and cells possess their own endogenous antioxidant defense system consisting primarily of three major enzymes: SOD, CAT, and GSH-Px. SOD serves to protect cells from toxicity by replacing superoxide ions with oxygen and hydrogen peroxide in normal condition (Case, 2017). It is known that almost all cells exposed to oxygen have antioxidant defense mechanisms. CAT is an enzyme that catalyzes the reaction in which hydrogen peroxide decomposes to form water and oxygen (Sofo et al., 2015). GSH-Px is an enzyme with the ability of peroxidase to protect cells from damage by oxygen (Xu et al., 2018). It reduces hydrogen peroxide to water (You and Park, 2011). Finally, MDA is a substance that occurs when ROS degraded polyunsaturated lipids, and is used mostly as an index to measure the level of oxidative stress (Bai et al., 2016).
The progression of apoptosis is known to reduce the activities of antioxidant enzymes such as SOD, CAT, and GSH-Px, and increase the levels of MDA. However, in this study, pretreatment with KGB was found to reverse the H2O2-induced reduction of antioxidant enzyme activity (SOD, CAT, and GSH-Px) and increase of MDA levels. These results suggest that KGB may mitigate oxidative damage to neurons induced by H2O2 by enhancing the activity of antioxidant enzymes and reducing intracellular lipid peroxidation levels.
Abnormalities in mitochondria can play a crucial role in apoptosis and the excessive production of ROS resulting from oxidative stress. These mitochondrial dysfunctions may lead to a decrease in MMP and other disturbances, ultimately leading to impaired cellular function. (Tian et al., 2015).
Furthermore, the mitochondria are involved in the production of free radicals and their accumulation in the cell body. (Dumont and Beal, 2011). In previous studies, oxidative stress induced by hydrogen peroxide reduces MMP, resulting in abnormal mitochondrial function. The current study revealed that H2O2-induced oxidative stress led to a significant increase in ROS production and mitochondrial dysfunction in the cells. However, pretreatment with KGB demonstrated a dose-dependent restoration of ROS levels and MMP in H2O2-treated SH-SY5Y cells. These findings suggest that KGB has the potential to protect SH-SY5Y cells against H2O2-induced cell death by inhibiting excessive ROS production and preventing mitochondrial dysfunction.
A previous study has demonstrated the association between mitochondria and apoptosis in H2O2-induced apoptosis in SH-SY5Y cells (de Oliveira et al., 2018), where the Bcl-2 protein family, including anti-apoptotic factor Bcl-2 and pro-apoptotic factor Bax, plays a crucial role in the regulation of mitochondrial membrane permeability (Choi et al., 2017). The interplay between BAX, caspase 9, and caspase 3 is crucial for the regulation and execution of apoptosis. BAX acts upstream of caspase 9 by inducing mitochondrial damage and cytochrome c release, which in turn activates caspase 9. Activated caspase 9 then activates caspase 3, leading to the cleavage of key cellular components and ultimately cell death (Saikumar et al., 2007; Malladi et al., 2009; Watanabe et al., 2018).
Our study revealed that KGB treatment increased the ratio of Bcl-2/Bax compared to H2O2-treated cells, and suppressed the expression and activation of caspase-3. These results suggest that KGB may regulate apoptotic proteins and act on the mitochondria-dependent apoptotic pathway.
Next, we examined MAP kinases (p38, JNK, and ERK1/2), one of the signaling pathways leading to apoptosis. The MAP kinase signaling pathway, which includes p38, JNK, and ERK1/2, has been proposed as a crucial mechanism in neurode-generative diseases mediated by oxidative stress (Kim and Choi, 2010; Oguchi et al., 2017).
Several studies have demonstrated the rapid activation of ERK1/2, JNK, and p38 by H2O2, all of which are strongly linked to ROS-induced cell death (Suzaki et al., 2002; Ruffels et al., 2004; Pan et al., 2013; Hu et al., 2015; Kim et al., 2015).
Our findings align with previous research indicating that H2O2 triggers the activation of MAPKs such as ERK1/2, JNK, and p38. Nevertheless, our results demonstrate that pretreatment with KGB effectively suppresses the phosphorylation of MAPKs induced by H2O2. This suggests that KGB possesses a protective effect against H2O2-induced oxidative stress by inhibiting MAPKs phosphorylation.
In summary, it has been confirmed that H2O2 induces cell death by increasing the oxidative environment within cells, breaking the balance of oxidation-reduction reactions in cells, and damaging cellular components such as proteins, mitochondria, and nucleic acids in SH-SY5Y cells. This study has demonstrated that KGB inhibits cell death by suppressing abnormal changes in cell death induced by oxidative stress.
These neuroprotective effects of KGB are mediated by its ability to restore antioxidant enzyme activities, decrease lipid peroxidation and ROS production, and improve MMP. Additionally, KGB exerts its protective effects by modulating the Bcl-2/Bax ratio, reducing caspase-3 levels, and suppressing MAPKs phosphorylation. These findings suggest that KGB may have potential as a as a therapeutic drug that can treat and prevent degenerative brain diseases caused by oxidative stress.
Acknowledgments
This research was supported by “Regional Innovation Strategy(RIS)” through the National Research Foundation of Korea(NRF) funded by the Ministry of Education(MOE) (2022RIS-005).
References
-
Bai WK, Zhang FJ, He TJ, Su PW, Ying XZ, Zhang LL and Wang T. (2016). Dietary probiotic Bacillus subtilis strain fmbj increases antioxidant capacity and oxidative stability of chicken breast meat during storage. PLoS One. 11:e0167339. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0167339, (cited by 2023 Jan 10).
[https://doi.org/10.1371/journal.pone.0167339]

-
Brieger K, Schiavone S, Miller Jr FJ and Krause KH. (2012). Reactive oxygen species: From health to disease. Swiss Medical Weekly. 142:w13659. https://smw.ch/index.php/smw/article/view/1569, (cited by 2022 Dec 3).
[https://doi.org/10.4414/smw.2012.13659]

-
Byun J, Kim SK and Ban JY. (2021). Anti-inflammatory and anti-oxidant effects of Korean ginseng berry extract in LPS-activated RAW264.7 macrophages. American Journal of Chinese Medicine. 49:719-735.
[https://doi.org/10.1142/S0192415X21500336]

-
Case AJ. (2017). On the origin of superoxide dismutase: An evolutionary perspective of superoxide-mediated redox signaling. Antioxidants. 6:82. https://www.mdpi.com/2076-3921/6/4/82, (cited by 2023 Jan 10).
[https://doi.org/10.3390/antiox6040082]

-
Cho IH, Kang BW, Park YJ, Lee HJ, Park S and Lee N. (2018). Ginseng berry extract increases nitric oxide level in vascular endothelial cells and improves cGMP expression and blood circulation in muscle cells. Journal of Exercise Nutrition and Biochemistry. 22:6-13.
[https://doi.org/10.20463/jenb.2018.0018]

-
Cho KS, Park CW, Kim CK, Jeon HY, Kim WG, Lee SJ, Kim YM, Lee JY and Choi YD. (2013a). Effects of Korean ginseng berry extract(GB0710) on penile erection: Evidence from in vitro and in vivo studies. Asian Journal of Andrology. 15:503-507.
[https://doi.org/10.1038/aja.2013.49]

-
Cho SY, Cho M, Seo DB, Lee SJ and Suh Y. (2013b). Identification of a small molecule activator of SIRT1 gene expression. Aging. 5:174-182.
[https://doi.org/10.18632/aging.100539]

-
Choi DW, Kim DK, Kanai Y, Wempe MF, Endou H and Kim JK. (2017). JPH203, a selective L-type amino acid transporter 1 inhibitor, induces mitochondria-dependent apoptosis in Saos2 human osteosarcoma cells. Korean Journal of Physiology and Pharmacology. 21:599-607.
[https://doi.org/10.4196/kjpp.2017.21.6.599]

-
de Oliveira MR, da Costa Ferreira G, Peres A and Bosco SMD. (2018). Carnosic acid suppresses the H2O2-induced mitochondria-related bioenergetics disturbances and redox impairment in SH-SY5Y cells: Role for Nrf2. Molecular Neurobiology. 55:968-979.
[https://doi.org/10.1007/s12035-016-0372-7]

-
Dey L, Xie JT, Wang A, Wu J, Maleckar SA and Yuan CS. (2003). Anti-hyperglycemic effects of ginseng: Comparison between root and berry. Phytomedicine. 10:600-605.
[https://doi.org/10.1078/094471103322331908]

-
Dumont M and Beal MF. (2011). Neuroprotective strategies involving ROS in Alzheimer disease. Free Radical Biology and Medicine. 51:1014-1026.
[https://doi.org/10.1016/j.freeradbiomed.2010.11.026]

-
He L, He T, Farrar S, Ji L, Liu T and Ma X. (2017). Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cellular Physiology and Biochemistry. 44:532-553.
[https://doi.org/10.1159/000485089]

-
Hu XL, Niu YX, Zhang Q, Tian X, Gao LY, Guo LP, Meng WH and Zhao QC. (2015). Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells. Environmental Toxicology and Pharmacology. 40:230-240.
[https://doi.org/10.1016/j.etap.2015.06.017]

-
Jung HW, Bae JH, Ko SK and Sohn UD. (2016). Ultrasonication processed Panax ginseng berry extract induces apoptosis through an intrinsic apoptosis pathway in HepG2 cells. Archives of Pharmacal Research. 39:855-862.
[https://doi.org/10.1007/s12272-016-0760-6]

-
Kim CK, Cho DH, Lee KS, Lee DK, Park CW, Kim WG, Lee SJ, Ha KS, Oh GT, Kwon YG and Kim YM. (2012). Ginseng berry extract prevents atherogenesis via anti-inflammatory action by upregulating phase II gene expression. Evidence-Based Complementary and Alternative Medicine. 2012:490301. https://www.hindawi.com/journals/ecam/2012/490301/, (cited by 2022 Dec 3).
[https://doi.org/10.1155/2012/490301]

-
Kim EK and Choi EJ. (2010). Pathological roles of MAPK signaling pathways in human diseases. Biochimica et Biophysica Acta. 1802:396-405.
[https://doi.org/10.1016/j.bbadis.2009.12.009]

-
Kim YJ, Kim JY, Kang SW, Chun GS and Ban JY. (2015). Protective effect of geranylgeranylacetone against hydrogen peroxide-induced oxidative stress in human neuroblastoma cells. Life Sciences. 131:51-56.
[https://doi.org/10.1016/j.lfs.2015.04.009]

-
Lee KS, Kim GH, Seong BJ, Kim SI, Han SH, Lee SS, Yang H and Yoo YC. (2014). Anti-inflammatory activity of solvent fractions from ginseng berry extract in LPS-induced RAW264.7 cells. Korean Journal of Medicinal Crop Science. 22:449-456.
[https://doi.org/10.7783/KJMCS.2014.22.6.449]

-
Malladi S, Challa-Malladi M, Fearnhead HO and Bratton SB. (2009). The Apaf-1·procaspase-9 apoptosome complex functions as a proteolytic-based molecular timer. EMBO Journal. 28:1916-1925.
[https://doi.org/10.1038/emboj.2009.152]

-
Mehendale SR, Wang CZ, Shao ZH, Li CQ, Xie JT, Aung HH and Yuan CS. (2006). Chronic pretreatment with American ginseng berry and its polyphenolic constituents attenuate oxidant stress in cardiomyocytes. European Journal of Pharmacology. 553:209-214.
[https://doi.org/10.1016/j.ejphar.2006.09.051]

-
Monteiro e Silva SA, Michniak-Kohn B and Leonardi GR. (2017). An overview about oxidation in clinical practice of skin aging. Anais Brasileiros de Dermatologia. 92:367-374.
[https://doi.org/10.1590/abd1806-4841.20175481]

-
Oguchi T, Ono R, Tsuji M, Shozawa H, Somei M, Inagaki M, Mori Y, Yasumoto T, Ono K and Kiuchi Y. (2017). Cilostazol suppresses Aβ-induced neurotoxicity in SH-SY5Y cells through inhibition of oxidative stress and MAPK signaling pathway. Frontiers in Aging Neuroscience. 9:337. https://www.frontiersin.org/articles/10.3389/fnagi.2017.00337/full, (cited by 2023 Feb 6).
[https://doi.org/10.3389/fnagi.2017.00337]

-
Pan LL, Liu XH, Jia YL, Wu D, Xiong QH, Gong QH, Wang Y and Zhu YZ. (2013). A novel compound derived from danshensu inhibits apoptosis via upregulation of heme oxygenase-1 expression in SH-SY5Y cells. Biochimica et Biophysica Acta. 1830:2861-2871.
[https://doi.org/10.1016/j.bbagen.2013.01.008]

-
Park HR, Lee HE, Park HY, Jeon JW, Cho WK and Ma JY. (2015). Neuroprotective effects of Liriope platyphylla extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. BMC Complementary and Alternative Medicine. 15:171. https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-015-0679-3, (cited by 2023 Jan 10).
[https://doi.org/10.1186/s12906-015-0679-3]

-
Ruffels J, Griffin M and Dickenson JM. (2004). Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: Role of ERK1/2 in H2O2-induced cell death. European Journal of Pharmacology. 483:163-173.
[https://doi.org/10.1016/j.ejphar.2003.10.032]

-
Saikumar P, Mikhailova M and Pandeswara SL. (2007). Regulation of caspase-9 activity by differential binding to the apoptosome complex. Frontiers in Bioscience. 12:3343-3354.
[https://doi.org/10.2741/2317]

-
Shao ZH, Xie JT, Vanden Hoek TL, Mehendale S, Aung H, Li CQ, Qin Y, Schumacker PT, Becker LB and Yuan CS. (2004). Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochimica et Biophysica Acta. 1670:165-171.
[https://doi.org/10.1016/j.bbagen.2003.12.001]

-
Singh A, Kukreti R, Saso L and Kukreti S. (2019). Oxidative stress: A key modulator in neurodegenerative diseases. Molecules. 24:1583. https://www.mdpi.com/1420-3049/24/8/1583, (cited by 2022 Nov 27).
[https://doi.org/10.3390/molecules24081583]

-
Sofo A, Scopa A, Nuzzaci M and Vitti A. (2015). Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. International Journal of Molecular Sciences. 16:13561-13578. https://www.mdpi.com/1422-0067/16/6/13561, (cited by 2023 Jan 20).
[https://doi.org/10.3390/ijms160613561]

-
Suzaki Y, Yoshizumi M, Kagami S, Koyama AH, Taketani Y, Houchi H, Tsuchiya K, Takeda E and Tamaki T. (2002). Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1(BMK1) and the MEF2C signaling pathway in PC12 cells: Potential role in cell survival following oxidative insults. Journal of Biological Chemistry. 277:9614-9621.
[https://doi.org/10.1074/jbc.M111790200]

-
Tan X, Gu J, Zhao B, Wang S, Yuan J, Wang C, Chen J, Liu J, Feng L and Jia X. (2015). Ginseng improves cognitive deficit via the RAGE/NF-κB pathway in advanced glycation end product-induced rats. Journal of Ginseng Research. 39:116-124.
[https://doi.org/10.1016/j.jgr.2014.09.002]

-
Tian X, Guo LP, Hu XL, Huang J, Fan YH, Ren TS and Zhao QC. (2015). Protective effects of Arctium lappa L. roots against hydrogen peroxide-induced cell injury and potential mechanisms in SH-SY5Y cells. Cellular and Molecular Neurobiology. 35:335-344.
[https://doi.org/10.1007/s10571-014-0129-7]

-
Uttara B, Singh AV, Zamboni P and Mahajan RT. (2009). Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology. 7:65-74.
[https://doi.org/10.2174/157015909787602823]

-
Watanabe R, Kurose T, Morishige Y and Fujimori K. (2018). Protective effects of fisetin against 6-OHDA-induced apoptosis by activation of PI3K-Akt signaling in human neuroblastoma SH-SY5Y cells. Neurochemical Research. 43:488-499.
[https://doi.org/10.1007/s11064-017-2445-z]

-
Xu Z, Regenstein JM, Xie D, Lu W, Ren X, Yuan J and Mao L. (2018). The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish and Shellfish Immunology. 72:564-571.
[https://doi.org/10.1016/j.fsi.2017.11.016]

-
Yi ES, Kim YJ, An YN, Han JA and Cho CH. (2018). Changes of berry characteristics and ginsenoside content depending on collection time of Korean ginseng berry. Korean Journal of Medicinal Crop Science. 26:214-219.
[https://doi.org/10.7783/KJMCS.2018.26.3.214]

-
You BR and Park WH. (2011). The effects of mitogen-activated protein kinase inhibitors or small interfering RNAs on gallic acid-induced HeLa cell death in relation to reactive oxygen species and glutathione. Journal of Agricultural and Food Chemistry. 59:763-771.
[https://doi.org/10.1021/jf103379d]

-
Yuan J, Chen Y, Liang J, Wang CZ, Liu X, Yan Z, Tang Y, Li J and Yuan CS. (2016). Component analysis and target cell-based neuroactivity screening of Panax ginseng by ultra-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. Journal of Chromatography B. 1038:1-11.
[https://doi.org/10.1016/j.jchromb.2016.10.014]

-
Zhang W, Cho SY, Xiang G, Min KJ, Yu Q and Jin JO. (2015). Ginseng berry extract promotes maturation of mouse dendritic cells. PLoS One. 10:e0130926. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0130926, (cited by 2022 Dec 3).
[https://doi.org/10.1371/journal.pone.0130926]
