Characterization of Antioxidant Activity and Anticancer Effect of an Endangered Plant, Lilium dauricum Ker-Gawl
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Lilium dauricum Ker-Gawl is an endangered plant, and it is highly valued as a medicinal plant owing to its excellent functionality.
L. dauricum leaf extract was shown to have high antioxidant activity and an inhibition concentration of 50% (IC50)value of 71.8 ± 0.13 ㎍/㎖ was shown for 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity. Its total phenol and flavonoid contents were 74.76 ± 0.24 ㎎·gallic acid equivalent (GAE)/g and 31.44 ± 0.27 ㎎·quercetin equivalent (QE)/g, respectively. Nitric oxide (NO) production was measured after oxidative stress was induced in lipopolysaccharide-stimulated RAW 264.7 cells, and 200 ㎍/㎖ of leaf extract effectively inhibited NO production at a rate of 17.84 ± 0.18%. Gastric cancer cells (AGS) and lung cancer cells (A549) were treated with 200 ㎍/㎖ of bulb extract, the inhibition rate for gastric cancer cell growth was 48.12 ± 2.60% whereas the inhibition rate for lung cancer cell growth was 55.83 ± 4.08%.
These results provide basic research data that can be used to identify the active constituents present in L. dauricum leaf and bulb extracts for the development of anticancer drugs, antioxidants, and functional products.
Keywords:
Lilium dauricum Ker-Gawl, Anticancer Effect, Antioxidant Activity, Nitric Oxide Production, Total Flavonoid Contents, Total Phenol ContentsINTRODUCTION
Exposure to various substances and environmental pollutants such as smoking, alcohol, and radiation have been exacerbated by industrialization. These environmental triggers generate unstable and highly reactive oxygen species (ROS) in the human body, which easily combine with various substances to synthesize oxidation products. These oxidized substances cause irreversible cell and tissue damage and promote toxicity and cancer cell formation (Lee et al., 2006).
Although the body maintains an antioxidant system that responds to ROS, a prolonged state of oxidative stress that exceeds the defense of this system can promote aging and chronic degenerative diseases such as cardiovascular disease, diabetes, cancer, and neurological diseases (Lee et al., 2016).
Therefore, to suppress the generation of free radicals in the living body and to lower their prevalence, the frequency of supplementary antioxidant intake has been increasing. Although there are synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BTT), they cause side effects when consumed above threshold levels. As a result, there has been increasing interest in the development of safe alternatives such as natural antioxidants derived from plant or food materials (Kumar et al., 2008; Ku and Kang, 2010).
Recent studies have been conducted to improve or treat various human diseases by identifying species with excellent antioxidant effects from edible or medicinal plants (Geronikaki and Gavalas, 2006). Natural antioxidant compounds are mainly polyphenols such as flavonoids, tannins, anthocyanins, carotenoids, and organic acids (Choi et al., 2015).
The safety of medicinal plants such as Astragalus propinguus, Simplicillium chinense, Lycium chinense, Glycyrrhiza uralensis, Chrysanthemum morifolium, Angelica gigas, Plantago ovate, and Cnidium officinale was also guaranteed, and studies on their antioxidant properties were conducted (Park, 2002; Kim et al., 2005; Park et al., 2005; Seo et al., 2008; Lee et al., 2011; Park et al., 2011).
Most of these antioxidant components are phenolic compounds of the flavonoid family. Representative anticancer substances derived from medicinal plants with antioxidant activity are taxol and vinblastine. Anticancer drugs obtained from these plants are reportedly more effective than synthetic anticancer drugs (Lee et al., 2016).
Clinical results showed that existing synthetic chemical products act on normal cells or tissues more than cancer cells, which may cause secondary infections such as the weakening of resistance due to the destruction of bone marrow cells and lymphocytes.
L. dauricum Ker-Gawl grows 1,400 m above sea level in the high mountainous regions of Gangwon-do, Jeollabuk-do, and Gyeongsangbuk-do, Korea. It grows to 1.5 m in height and has narrow wings on the stem. Underground scales were white, round, and 3 ㎝ - 5 ㎝ in diameter. The lanceolate leaves are 5 ㎝ - 12 ㎝ long and run alternately without petioles, and three to five veins have fine processes along the edges. It has upward-facing flowers from July to August, and the yellow-red flowers run upward in an umbel at the end of the main stem. Inflorescences are 7 ㎝ - 8 ㎝ long, six in number, broadly lanceolate, spread obliquely, and the tip is slightly bent back. Purple spots appeared around the inside of the flowers. The fruit has a narrow, obovate capsule. L. dauricum grows only in high mountains, and because of its beautiful flower shape and color, it has a very high ornamental value and is of great value as a genetic resource for cultivating new varieties.
However, it is currently considered an endangered species on the Korean Red List and is in danger of extinction owing to the destruction of its native habitat and indiscriminate harvesting for ornamental purposes (Kim, 2020).
Research into potential uses and functions has been limited this study, extracts were prepared for each tissue part to examine the antioxidant activity by measuring total phenol and flavonoid contents, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging ability. The potential of L. dauricum as a new functional food material was also assessed. In addition, we aimed to demonstrate the inhibitory effect of L. dauricum extract on the proliferation of human cancer cells.
MATERIALS AND METHODS
1. Plant material
To analyze biological activity, L. dauricum was acclimatized to aseptic culture using plant cell tissue culture technology. As for the acclimatization method, aseptic plants planted in the medium were adapted to the soil for gardening after removing the medium. After 12 weeks of acclimatization, the plants were separated into bulbs, stems, leaves, petals and pistil and stamens and used for extraction and concentration experiments (Fig. 1).
2. Extraction and concentration
The L. dauricum used in this experiment was washed cleanly, dried in the shade, and then ground with blender and used as the solvent, and 70% EtOH was used. Phenolic compounds were extracted using maceration technique with modification.
Each sample (10 g rhizome, 10 g stem, 10 g leaf, 5 g flower, 2 g pistil and stamens) added 20 times the solvent was extracted after sonication for 30 minutes. The extraction process was repeated 3 times, and after extraction, it was concentrated under reduced pressure (EYELA N-1000, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) and used in the experiment.
3. Antioxidant activity assay by DPPH free radical scavenging
The DPPH radical scavenging activity assay of the extracts from each part of L. dauricum was performed by partially modifying the method described by Blois (1958).
After mixing 0.1 ㎖ of the sample with 0.1 ㎖ of 0.15 mM DPPH and reacting at room temperature for 30 min, absorbance was measured at 517 ㎚ with a UV/VIS spectrophotometer (V530, Jasco Co., Tokyo, Japan) to test the activity.
Ascorbic acid was used as a positive control, and the DPPH radical scavenging ability according to the concentration change was expressed as inhibitory concentration (IC50) and obtained as a slope to measure the sample concentration at which the scavenging ability was 50%.
4. Determination of total phenol and flavonoid contents
The total phenol content was measured by modifying the method of Folin-Ciocalteu using the general oxidation-reduction properties of phenolic substances (Singleton and Rossi, 1965).
After mixing 0.1 ㎖ of the sample with 0.05 ㎖ of Folin-Ciocalteu reagent, 0.3 ㎖ of 20% sodium carbonate was added. The solution was then stabilized at 20℃ for 15 min, and the absorbance was measured at 725 ㎚ (Multiskan FC Microplate Photometer, Thermo Fisher Scientific Inc., Waltham, MA, USA) by adding 1 ㎖ of distilled water.
A standard calibration curve was prepared by treating gallic acid used as a standard at a concentration of 0 ㎎/㎖ - 100 ㎎/㎖, and the phenolic compounds of the sample were quantified and expressed as gallic acid equivalent (GAE).
Total flavonoid content was measured by partially modifying the method described by Moreno et al. (2000). Solutions of 0.1 ㎖ of a sample diluted with 80% ethanol, 0.02 ㎖ of 1 M potassium acetate, 0.02 ㎖ of 10% aluminum chloride, and 0.86 ㎖ of 80% ethanol were combined, reacted at room temperature for 40 min, and absorbance was measured at 415 ㎚ (Multiskan FC Microplate Photometer, Thermo Fisher Scientific Inc., Waltham, MA, USA). Total flavonoid content was quantified using a quercetin calibration curve and expressed as quercetin equivalents (QE).
5. Cytotoxicity measurement
The cytotoxicity of the sample was measured by the 3-(4,5-dimethy-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method, which was partially modified from the method of Mosmann et al. (1983).
RAW264.7 cells were seeded in a 96-well plate at 1 × 105 cells/well and cultured in a CO2 incubator for 24 h. After adding samples and treatment with LPS at a concentration of 4 ㎎/ℓ, the solution was incubated for an additional 24 h. MTT dissolved in phosphate-buffered saline (PBS) was added at a concentration of 0.5 ㎎/㎖, the supernatant was removed, and absorbance was measured at 540 ㎚ by treatment with 100 ㎕ of dimethyl sulfoxide.
6. Measurement of nitric oxide (NO) production rate
NO, an ROS, was measured using Griess reagent. RAW264.7 cells were seeded in a 96-well plate at 1 × 105 cells/well and cultured in a CO2 incubator for 24 h. The lipopolysaccharide (LPS) and samples were added and cultured for an additional 24 h. Then, 50 ㎕ of the supernatant was mixed with 50 ㎕ of Griess reagent (A reagent: 1% sulfanilamide, B reagent: 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride dissolved in 5% phosphoric acid), and absorbance was measured at 540 ㎚.
7. Measurement of anti-cancer activity
All cells used in the study were purchased from the Korea Cell Line Bank (Seoul, Korea).
For the anti-cancer activity assay, all cells related to cancer were cultured for 24 h in a CO2 incubator using a Dulbecco's Modified Essential Medium (DMEM) medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic (penicillin, 100 U/㎖). Normal cells (HEK 293) and human cancer cells including cervical cancer cells (HeLa), lung cancer cells (A549), gastric cancer cells (AGS), prostate cancer cells (PC-3) were used in anticancer experiments.
In the DMEM medium supplemented with 10% FBS and 1% antibiotics (penicillin, 100 U/㎖), HeLa and HEK 293 were cultured. AGS, PC-3, and A549 were cultured in a CO2 incubator using Roswell Park Memorial Institute Medium (RPMI) medium supplemented with 10% FBS and 1% antibiotic (penicillin, 100 U/㎖).
8. Data analysis
All data were represented as mean ± standard deviation by performing at least three repetitions, and the software used for statistical analysis was IBM SPSS Statistics V26 program (SPSS Inc., Chicago, IL, USA). Variation analysis was analyzed for statistical significance at the 5% level using Duncan’s Multiple Range Test (DMRT, p < 0.05).
RESULTS
1. Antioxidant activity of the leaf tissue
The antioxidant activity was measured using the DPPH scavenging method by extracting bulb, stems, leaves, petals and pistil and stamens for each part. The antioxidant activity of the leaf extract was 71.8 ± 0.13 ㎍/㎖, indicating the highest antioxidant activity (Table 1).

The total phenolic, flavonoid contents and DPPH radical scavenging activity of extracts from different parts in L. dauricum.
The lowest radical scavenging ability was 263.29 ± 5.67 ㎍/㎖ in a pistil and stamens extract. The total phenol content of each extract was measured and ranked in descending order: Leaf (74.76 ± 0.24 ㎎·GAE/g); flowers (68.46 ± 0.53 ㎎·GAE/g); pistil and stamens (53.46 ± 0.59 ㎎·GAE/g); stem (32.2 ± 0.24 ㎎·GAE/g); and rhizome (27.32 ± 0.44 ㎎·GAE/g).
The total flavonoid content also showed the highest value in the leaf extract at 31.44 ± 0.27 ㎎·QE/g. The correlation between the DPPH scavenging ability, total phenol content, and total flavonoid content was shown, suggesting that the overall antioxidant activity of the leaf was high.
2. Anti-inflammatory activity of the leaf tissue
RAW264.7 cells were treated with extracts of each part of L. dauricum at various concentrations (10, 50, 100, and 200 ㎍/㎖), and cell viability was investigated.
No toxicity was observed at concentrations below 200 ㎍/㎖ (Fig. 2A). Accordingly, the extracts for each part of L. dauricum were treated at a concentration of 200 ㎍/㎖ or less, and LPS stimulation was applied to measure the production of NO, which is an inflammatory sub-stance. NO production decreased as the concentration increased in the treatment group, except for the flower extract (Fig. 2B).
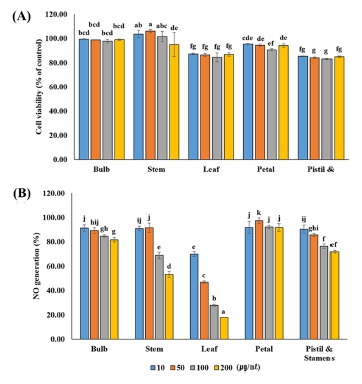
Cell viability (A) by MTT assay and NO production (B) in LPS-induced Raw 264.7 cell with extractions obtained from various tissue parts of L. dauricum.This value was performed to triplicate and represented as means ± standard deviation. Statistical significance was indicated by different letters at the 5% level by Duncan’s Multiple Range Test (DMRT) analysis (p < 0.05).
At 200 ㎍/㎖ of the leaf extract, each showed an NO production rate of 17.84 ± 0.18%. As a result of statistically significant by tissue parts, the leaf extract of L. dauricum was confirmed as a potential biomaterial for inflammation drug development.
3. Anticancer activity of the bulb extract
To check the presence or absence of anticancer activity of extracts from each tissue part of L. dauricum, the anticancer activity was tested using gastric cancer cells (AGS), lung cancer cells (A549), prostate cancer cells (PC-3), and cervical cancer cells (HeLa).
The cell viability of extracts of various concentrations of L. dauricum was measured using HEK 293 cells. Most showed a survival rate of more than 90% and showed no toxicity, however, to have less effect on cells in relation to cell osmotic pressure, the anticancer activity test was conducted at 200 ㎍/㎖ or less for each extract (Fig. 3A).
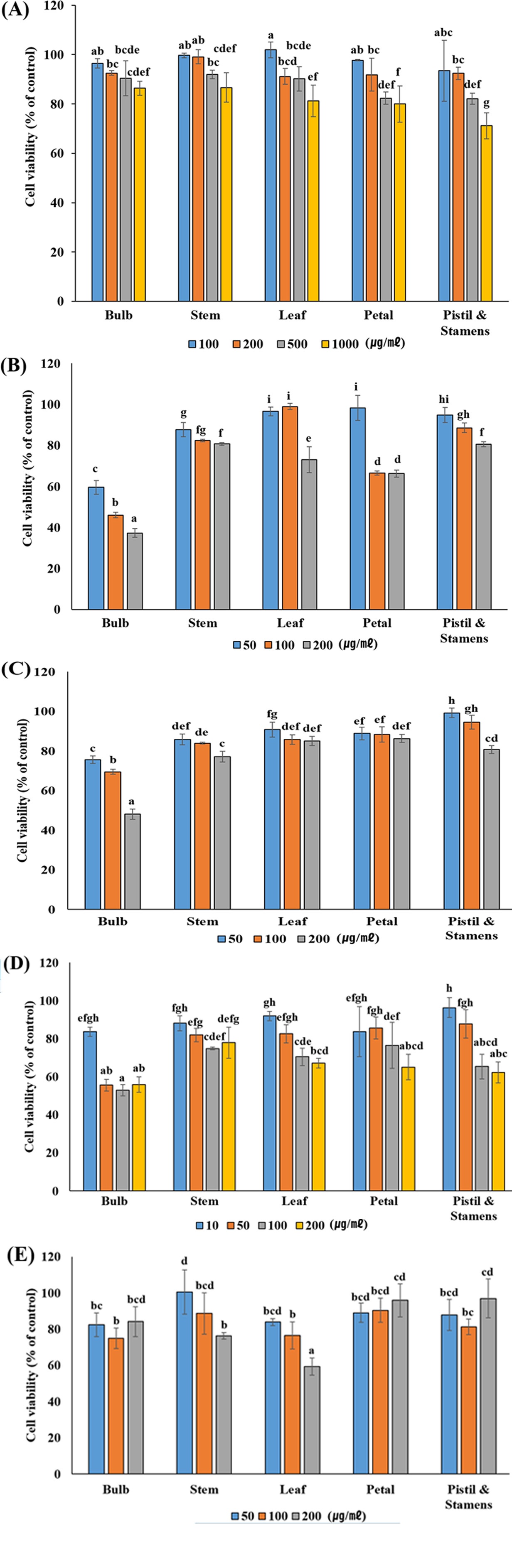
Cell viability by MTT assay in HEK293 cell (A) and inhibitory effect of extractions obtained from various tissue parts of L. dauricum on proliferation of HeLa (B: Cervical cancer), AGS (C: Gastric cancer), A549 (D: Lung cancer) and PC-3 (E: Prostate cancer) cells.This value was performed to triplicate and represented as means ± standard deviation. Statistical significance was indicated by different letters at the 5% level by Duncan’s Multiple Range Test (DMRT) analysis (p < 0.05).
When the bulb extract, which showed low antioxidant activity, was treated with different concentrations (50, 100, and 200 ㎍/㎖) of HeLa cells, the inhibition rates of cancer cell growth were statistically significant, 59.53%, 46.09%, and 37.28%, respectively. (Fig. 3B).
In addition, when AGS cells were treated with bulb extract at a concentration of 200 ㎍/㎖, the survival rate was 48.12%, indicating a high cancer cell growth inhibition rate (Fig. 3C), and A549 cells were also treated with the bulb extract of a concentration of 200 ㎍/㎖. The mean survival rate was 55.83%, and cancer cell growth was inhibited (Fig. 3D). The inhibition rate for cancer cell growth of AGS and A549 was the highest in the bulb extract, which was statistically significant. The PC-3 cells showed the highest rate of 59.32% in anticancer activity when 200 ㎍/㎖ of L. dauricum leaf extract was treated (Fig. 3E).
DISCUSSION
Oxidative stress is known to cause various diseases by causing an imbalance between pro-oxidants and antioxidants (Bengmark et al., 2009). For example, in a patient suffering from chronic cardiovascular disease, a large amount of free radicals is produced and cause fatal damage to cells or tissues (Lee et al., 2003; Geronikaki and Gavalas, 2006; Kondo et al., 2009).
Therefore, to prevent or treat chronic and inflammatory diseases, the activity of antioxidant enzymes such as SOD (superoxide dismutase), catalase, and glutathione peroxidase as well as external natural substances such as vitamins C and E, selenium, polyphenols, and flavonoids must be maintained. Recently, studies on the discovery of antioxidants derived from natural products that can effectively remove active oxygen, such as superoxide anions (O2−), H2O2, ONOO− and NO, have been conducted (Kumar et al., 2008).
The main goal of this study was to evaluate the antioxidant potential and anticancer activity of L. dauricum. However, the total antioxidant capacity of individual plants was affected by various phytochemicals in plant tissues or cells, and this potential could not be accurately assessed using a single procedure (Chu et al., 2000).
Therefore, we determined the overall antioxidant capacity of L. dauricum using various methods including a DPPH assay, total phenol content, and total flavonoid content to standardize the activity characteristics of each tissue extract.
In this experiment, the antioxidant capacity of the leaf tissue extract of L. dauricum was found to be much higher than that of the other tissue parts, including bulb, stem, petal and pistil and stamens. The total flavonoid content in the leaf extract was 31.44 ± 0.27 ㎎·QE/g, which was 4 - 5 times higher than that of other tissue extracts. The leaf extract also showed the highest total phenol content (74.76 ± 0.24 ㎎·GAE/g) and the highest antioxidant activity as determined using the DPPH method.
A study by Mok et al. (2011) showed that the total polyphenol and flavonoid contents were found to be the highest in the leaf and flower tissue extracts, showing a similar trend to our results. Polyphenols and flavonoids are widely distributed throughout the plant kingdom. Polyphenol compounds have two or more hydroxyl groups, and flavonoid compounds are aromatic compounds with a basic skeleton of C6-C3-C6, which easily bind to proteins (Yu et al., 2006).
Flavonoids with flavones as a basic structure are abundantly present in the flowers, stems, and fruits of plants and reportedly have antioxidant, anticancer, and anti-inflammatory effects (Vijaya et al., 1995). As described above, this study shows that the leaf-derived antioxidant activity of L. dauricum is excellent because it contains flavonoids and phenols.
Most human diseases involve a degree of inflammation; therefore, drugs with anti-inflammatory effects are in constant development. These anti-inflammatory drugs are generally based on synthetic materials; however, natural plant materials may minimize side effects and provide alternative treatments.
This study determined that for L. dauricum, the leaf tissue extract had the highest anti-inflammatory effect compared to tissue extracts from the other plant parts, including bulb, stems, petal, pistils, and stamens. In a study of the anti-inflammatory properties of C. japonicum, the leaf extract, which had the best antioxidant activity, was reported to significantly inhibit NO production by effectively inhibiting the expression of iNOS and COX-2 in LPS-stimulated RAW264.7 macrophages (Mok et al., 2011). Another study examined the anti-inflammatory effects of T. kirilowii and showed that the NO production inhibitory ability was ranked in the order of seed, fruit, and flower extracts (Park and Kang, 2016).
In the present study of L. dauricum, the leaf extract had high antioxidant activity and a high anti-inflammatory effect. Similar results were reported in studies of C. japonicum. The exact relationship between antioxidant activity and anti-inflammatory effect may vary depending on the plant species and the parts these tissue extracts are obtained.
Medicinal plants have been used for centuries because of their proven therapeutic effect on anticancer activity. As many compounds isolated from plants exist in a mixed form, the anticancer activity of plant extracts has been extensively studied (Liu, 2003; Karna et al., 2012).
Studying secondary metabolites in plant component mixtures is important in determining the biological effects of medicinal plants. The effect of the tissue extract varies depending on the plant part (Reichelt et al., 2002; Chen et al., 2003). This study demonstrated that the anticancer activity of the tissue extracts for each part of L. dauricum was different.
The cancer cell suppression activity of bulb extract against cervical, gastric, and lung cancer cells was found to have a significant effect compared to other tissue extracts. However, the anticancer activity of the leaf tissue extract of L. dauricum was found to have the highest efficacy in the inhibition of prostate cancer cells.
For Trapa japonica, the cancer cell proliferation inhibitory activity of the pericarp extract was more effective than that of the seed extract, which was assessed using a total of 7 cancer cell lines, including A549, AGS, HeLa, PC-3, HCT-116, HT29, SW620 (Han et al., 2016).
Lee et al. (2016) studied the inhibitory effects of E. japonica seed, flesh, and leaf ethanol extracts on liver cancer cells (H460), gastric cancer cells (AGS), and lung cancer cells (A549) and showed that seed extracts yielded the optimal results.
Many anticancer studies have tested whole plant extracts instead of isolated plant components and compounds. There are currently only a few studies on the anticancer mechanisms of the components of L. dauricum. Therefore, additional in-depth research on the development of natural materials from endangered plants is required. Tissue extracts from the leaves and bulbs of L. dauricum show excellent biological activity and should be used as a starting point for the development of pharmaceutical materials related to antioxidant and cancer cell inhibition agents and functional foods.
Limited research on the physiological activity mechanism of L. dauricum has been previously reported, despite the high scarcity value of this endangered plant. This study determined the antioxidant activities of tissue in separate plant parts of L. dauricum and examined whether the highest antioxidant activity was also related to the highest anti- inflammatory and anticancer activities.
For example, the leaf extract, which had high antioxidant activity, also showed good anti-inflammatory activity with high total phenol and flavonoid contents. The leaf extract also showed good cancer cell suppression activity in prostate cancer cells. Bulb tissue extract displayed significant inhibitory activity against cervical cancer, gastric cancer, and lung cancer. Each separate extract corresponding to a separate part of L. dauricum may express the mechanism for anticancer activity differently.
This shows considerable potential for developing future anticancer and anti-inflammatory treatments related to active oxygen scavenging ability.
Acknowledgments
This study was supported by the Gangwondo Nature Environment Research Park and Gangwondo Forest Science Institute.
References
- Bengmark S, Mesa MD and Gil A. (2009). Plant-derived health: The effects of turmeric and curcuminoids. Nutrición Hospitalaria. 24:273-281.
-
Blois MS. (1958). Antioxidant determinations by the use of a stable free radical. Nature. 181:1199-1200.
[https://doi.org/10.1038/1811199a0]

-
Chen F, Tholl D, D'Auria JC, Faroop A, Pichersky E and Gershenzon J. (2003). Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. The Plant Cell. 15:481-494.
[https://doi.org/10.1105/tpc.007989]

-
Choi MH, Jeon YJ and Shin HJ. (2015). Anthocyanin analysis of pressure-extracted korean blueberry juice and in vitro antiinflammatory in RAW267.4 cell line. Korean Society for Biotechnology and Bioengineering Journal. 30:191-196.
[https://doi.org/10.7841/ksbbj.2015.30.4.191]

-
Chu YH, Chung HC and Hsu HF. (2000). Flavonoid content of several vegetables and their antioxidant activity. Journal of the Science of Food and Agriculture. 80:561-566.
[https://doi.org/ 10.1002/(SICI)1097-0010(200004)80:5<561::AID-JSFA574>3.0.CO;2-%23]

-
Geronikaki AA and Gavalas AM. (2006). Antioxidants and inflammatory disease: Synthetic and natural antioxidants with anti-inflammatory activity. Combinatorial Chemistry and High Throughput Screening. 9:425-442.
[https://doi.org/10.2174/138620706777698481]

-
Han HM, Kwon YS and Kim MJ. (2016). Antioxidant and antiproliferative activity of extracts from water chestnut(Trapa japonica Flerow). Korean Journal of Medicinal Crop Science. 24:14-20.
[https://doi.org/10.7783/KJMCS.2016.24.1.14]

-
Karna P, Chagani S, Gundala SR, Rida PCG, Asif G, Sharma V, Gupta MV and Aneja R. (2012). Benefits of whole ginger extract in prostate cancer. British Journal of Nutrition. 107:473-484.
[https://doi.org/10.1017/S0007114511003308]

- Kim HK. (2020). In vitro mass propagation and biological activities of Lilium dauricum Ker Gawl. Ph. D. Thesis. Kangwon National University. p.2-3.
- Kim SK, Ban SY, Kim JS and Chung SK. (2005). Change of antioxidant activity and antioxidant compounds in Saururus chinensis by extraction conditions. Journal of Applied Biological Chemistry. 48:89-92.
-
Kondo T, Hirose M and Kageyama K. (2009). Roles of oxidative stress and redox regulation in atherosclerosis. Journal of Atherosclerosis and Thrombosis. 6:532-538.
[https://doi.org/10.5551/jat.1255]

-
Ku KM and Kang YH. (2010). Antioxidant and quinone reductase inductive activities of various organs of pepper. Journal of Applied Biological Chemistry. 53:31-36.
[https://doi.org/10.3839/jabc.2010.006]

-
Kumar S, Kuma D, Manjusha, Saroha K, Singh N and Vashishta B. (2008). Antioxidant and free radical scavenging potential of Citrullus colocynthis(L.) Schrad. methanolic fruit extract. Acta Pharmaceutica. 58:215-220.
[https://doi.org/10.2478/v10007-008-0008-1]

-
Lee H, Kim YK, Lee HJ and Lee JJ. (2016). Effects of loquat(Eriobotrya japonica Lindl.) ethanol extracts of different aerial parts on antioxidant activity and antiproliferation of human cancer cells. Korean Journal of Community Living Science. 27:211-220.
[https://doi.org/10.7856/kjcls.2016.27.2.211]

- Lee HK, Kim JS, Kim NY, Kim MJ, Park SU and Yu CY. (2003). Antioxidant, antimutagenicity and anticancer activities of extracts from Circium japonicum var. ussurience Kitamura. Korean Journal of Medicinal Crop Science. 11:53-61.
- Lee JL, KIM SW, Yoo YK, Lee GT and Lee KK. (2006). Antiwrinkle effect of Morinda citrifolia(noni) extracts. Journal of the Society of Cosmetic Scientists of Korea. 32:227-231.
- Lee KJ, Park MH, Park YH, Lim SH, Kim KH, Kim YG, Ahn YS and Kim HY. (2011). Antioxidant activity and nitric oxide production of ethanol extracts from Astragali membranaceus Bunge and A. membranaceus Bunge var mongholicus Hisiao. Journal of the Korean Society of Food Science and Nutrition. 40:1793-1796.
- Liu RH. (2003). Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. American Journal of Clinical Nutrition. 78:517S-520S.
- Mok JY, Kang HJ, Cho JK, Jeon IH, Kim HS, Park JM, Jeong SI, Shim JS and Jang SI. (2011). Antioxidative and anti-inflammatory effects of extracts from different organs of Cirsium japonicum var. ussuriense. Korea Journal of Herbology. 26:39-47.
- Moreno MIN, Isla MI, Sampietro AR and Vattuone MA. (2000). Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. Journal of Ethnopharmacology. 71:109-114.
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 65:55-63.
- Park BH, Cho HS and Kim DH. (2005). Antioxidative effects of solvent extracts of Lycii fructus powder(LFP) and Maejakgwa made with LFP. Journal of the Korean Society of Food Science and Nutrition. 34:1314-1319.
- Park MJ and Kang YH. (2016). Anti-oxidant and anti-inflammatory activities of various organ extracts from Trichosanthes kirilowii Maxim. Korean Journal of Pharmacognosy. 47:327-332.
- Park SJ, Yoon JH, Kim YE, Yoon WB and Kim JD. (2011). In vitro antioxidant activity of the aqueous of Angelicae gigas Nakai leaves. Korean Journal of Food Preservation. 18:817-823.
- Park YS. (2002). Antioxidative activities and contnets of polyphenolic compound of medicinal herb extracts. Journal of the East Asian Society of Dietary Life. 12:23-31.
- Reichelt M, Brown PD, Schneider B, Oldham NJ, Stauber E, Tokuhisa J, Kliebenstein D, Mitchell-Olds T and Gershenzan J. (2002). Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry. 59:663-671.
- Seo HS, Chung BH and Cho YG. (2008). Antioxidant and anticancer effects of agrimony(Agrimonia pilosa L.) and Chinese lizardtail (Saururus chinensis Baill). Korean Journal of Medicinal Crop Science. 16:139-143.
- Singleton VL and Rossi JA. (1965). Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16:144-158.
- Vijaya K, Ananthan S and Nalini R. (1995). Antibacterial effect of the aflavin, polyphenon 60(Camellia sinensis) and Euphorbia hirta on Shigella spp. - a cell culture study. Journal of Ethnopharmacology. 49:115-121.
- Yu MH, Im HG, Lee HJ, Ji YJ and Lee IS. (2006). Components and their antioxidative activities of methanol extracts from sarcocarp and seed of Zizyphus jujuba var. inermis Rehder. Korean Journal of Food Science and Technology. 38:128-134.