Morphological Variation of Cultivated and Weedy Types in Perilla Crop Collected from South Korea
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Perilla (Perilla frutescens L.) has been widely cultivated and used for medicinal use, aromatic, functional food, and ornamental plant in South Korea. To understand the morphological variation in the cultivated and weedy types of Perilla (CWTP) collected from South Korea, 52 accessions collected from different areas were evlauated using one quantitative and 10 qualitative characteristics.
Principal component analysis (PCA) was performed using the NTSYS-pc V2.1 program to detect the differences among the accessions of the CWTP. Multivariate analysis was performed using Microsoft Excel Statistical Analysis System. The cultivated P. frutescens var. frutescens and two weedy types of Perilla crop were accurately distinguished based on he 11 morphological characteristics, paricularly seed related traits, used in the study. PCA results showed that, most morphological characteristics such as weight per 100 seeds (QN1), color of leaf adaxial side (QL1), color of leaf abaxial side (QL2), stem color (QL4), flower color (QL5), seed size (QL9), and seed hardness (QL10) provided a significant contribution in the positive or negative direction on the first axis. These characteristics are considered useful for distinguishing between accessions of the CWTP collected from South Korea.
The accessions of weedy P. frutescens var. frutescens are located between the accessions of cultivated P. frutescens var. frutescens and the those of weedy P. frutescens var. crispa on the first axis. Although the cultivated and weedy types of P. frutescens var. frutescens and the two weedy types of P. frutescens var. frutescens and P. frutescens var. crispa cannot be completely distinguished, the results provide useful information for future studies on the identification and classification of CWTP germplasm accessions collected from different areas in South Korea.
Keywords:
Perilla Crop, Cultivated and Weedy Types, Morphological Characteristics, Morphological Differentiation, Crop Evolution, Principal Component AnalysisINTRODUCTION
Perilla (Perilla frutescens L.) is an annual crop of the Lamiaceae family, and it can be divided into two cultivated types (or varieties) based on their morphological characteristics and usage conditions: P. frutescens var. frutescens and P. frutescens var. crispa (Makino, 1961).
This crop is considered to have originated in East Asia, including China, because in East Asia there is a long history of cultivation, large scale distribution and cultivation, and a wide range of uses (Makino, 1961; Li, 1969; Nitta, 2001; Nitta et al., 2003).
Today, the two cultivated types of Perilla crop are most widely cultivated and used in East Asia (Li, 1969; Lee and Ohnishi, 2001; Lee and Ohnishi, 2003; Nitta et al., 2003) as follows: The cultivated var. frutescens is widely cultivated in South Korea, where it is mainly used for both oil and vegetable crop; and the cultivated var. crispa is widely cultivated in Japan, where it is mainly used for both herbal medicine and vegetable crop (Lee and Ohnishi, 2001; Nitta, 2001; Lee and Ohnishi, 2003; Nitta et al., 2003).
In recent years, Perilla has attracted worldwide attention as a multi-purpose cash crop with uses such as for oil, condiment, vegetable, herbal medicine, and ornamental plants (Park et al., 2021).
In particularly, Perilla leaves are rich in iron, vitamin B and C, and anthocyanin, and are preferred in salad vegetables and pickles eaten with meat and sashimi in South Korea and Japan (Lee and Ohnishi, 2001; Nitta et al., 2003).
Also, Perilla seed oil of P. frutescens var. frutescens contains polyunsaturated fatty acids such as ω-3 fatty acids (alpha-linolenic acid), ω-6 fatty acids (linoleic acid) and ω-9 fatty acids (Oleic acid), which are the most beneficial to human health in prevention and control of various diseases like cardiovascular disorders, cancer, inflammation, rheumatoid arthritis, and mental health condition (Asif, 2011; Hashimoto et al., 2020; Park et al., 2021).
Since the morphological characteristics of the two cultivated types of Perilla differ in many respects, the two cultivated types of Perilla are well differentiated based on morphological characteristics. Of these, the cultivated var. frutescens has large (more than 2 ㎜) soft seeds, green leaves and stems, and leaves without wrinkles. In contrast, the cultivated var. crispa has small (less than 2 ㎜) hard seeds, purple or green coloration on the leaves and stems, and wrinkly or non-wrinkly leaves (Makino, 1961; Lee and Ohnishi, 2001; Nitta, 2001; Nitta et al., 2003).
In addition, although the wild species of the two cultivated types of Perilla has not been identified in East Asia, its weedy types are commonly found in East Asia including in China and Korea in habitats such as wasteland, roadside, and around farmers' houses or farmers’ fields (Lee and Ohnishi, 2001; Lee et al., 2002; Lee and Ohnishi, 2003; Nitta et al., 2003; Nitta et al., 2005; Sa et al., 2015; Ma et al., 2017; Sa et al., 2018; Ma et al., 2019; Ha et al., 2021).
Until now, many plant researchers have been conducting research to distinguish between the two cultivated and weedy types of Perilla (CWTP). Compared with the two cultivated types of Perilla, the weedy types are relatively indistinguishable based on morphological characteristics.
The two cultivated types of Perilla have the same number of chromosomes (2n = 40) (Yamane, 1950; Honda et al., 1994), they can be crossed by artificial crossing, and intermediate hybrid types exist in the natural habitat (Nagai, 1935; Honda et al., 1990, 1994; Lim et al., 2019, 2021; Kim et al., 2021). The presence of intermediate hybrid types is the reason why the two CWTP are difficult to distinguish completely (Lee and Ohnishi, 2001).
Modern biotechnology is now mainstream, and various molecular marker technologies such as random amplified polymorphic DNA (RAPD) (Nitta et al., 2003; Nitta and Ohnishi, 1999), amplified fragment length polymorphism (AFLP) (Lee and Ohnishi, 2003; Lee et al., 2002), and simple sequence repeats (SSR) (Park et al., 2009; Sa et al., 2013, Sa et al., 2018; Ha et al., 2021; Kim et al., 2021; Park et al., 2021; Sa et al., 2021; Fu et al., 2022; Park et al., 2022) have been applied successfully to identify and classify germplasm accessions of the CWTP.
However, the identification and description of phenotypic morphological traits with obvious and easily identifiable characteristics are still the most direct and basic methods and means of germplasm research, and they cannot be replaced completely by molecular marker technology (Liao et al., 2015).
Unlike China, which has a long history of cultivation and high genetic diversity of Perilla crop but few uses, South Korea, which has large-scale cultivation and multi-purpose use, is considered to be a secondary diffusion center of Perilla crop (Li, 1969; Lee and Ohnishi, 2001; Lee and Ohnishi, 2003; Nitta et al., 2003; Nitta et al., 2005).
In order to study and utilize comprehensively the Perilla crop germplasm resources collected from South Korea, it is necessary to identify and evaluate a variety of morphological characteristics of these germplasm resources, which will provide a research basis for future research and utilization of Perilla germplasm resources.
Therefore, in order to understand further the morphological variation among accessions of the CWTP, in this study we investigated the morphological characteristics of 52 accessions of the CWTP from South Korea using one quantitative and 10 qualitative characteristics. This study is expected to provide useful information for future taxonomic studies of CWTP germplasm accessions collected from South Korea.
MATERIALS AND METHODS
1. Plant materials
The 52 accessions of the CWTP (21 cultivated var. frutescens, 14 weedy var. frutescens, 17 weedy var. crispa) used in this study were selected from a previous study by Fu et al. (2022). In the present study, the 52 accessions of the CWTP used represented all regions of South Korea as much as possible, and the collection information is shown in Table 1. The subset of each collections was deposited in the National Agrobiodiversity Center, National Institute of Agricultural Sciences, Rural Development and Administration, Suwon, Republic of Korea, for permanent seed preservation.
2. Morphological characteristic survey
To evaluate the morphological variation between the Perilla and related weedy types from South Korea, the 52 accessions of the CWTP used for morphological investigation in this study were selected from germplasm accessions used for genetic variation analysis in a previous study by Fu et al. (2022).
To investigate the morphological characteristics, 10 seeds of each accession were sown in a seedling box at the end of May 2022 and kept in a glass greenhouse for one month. Then in early July 2022, five seedlings of each accession were transplanted into a field at the experimental farm of Kangwon National University.
One quantitative and 10 qualitative characteristics of each Perilla accession used in this study were selected on the basis of a previous report by Lee and Ohnishi (2001), and the specific information is shown in Table 2.
3. Data analysis
Principal component analysis (PCA) was performed using the NTSYS-pc V2.1 Program (Rohlf, 1998) to detect the differences among accessions of the CWTP.
Multivariate analysis was performed using the Microsoft Excel Statistical Analysis System program. IBM SPSS Statistics version 21 (IBM Co., Armonk, N.Y., USA) software was used to perform correlation analysis for the one quantitative and 10 qualitative characteristics in the 52 accessions of the CWTP.
RESULTS
1. Morphological characteristics among accessions of the CWTP collected from South Korea
The results of a survey of the morphological characteristics of the one quantitative and 10 qualitative characteristics of the 52 accessions of the CWTP collected from South Korea are shown in Table 3.

Mean values, standard deviation, range, and accession number for one quantitative and 10 qualitative characteristics among 52 accessions of the CWTP collected from different areas of South Korea.
According to the survey results of the one quantitative trait, the average weight per 100 seeds (QN1) for the cultivated var. frutescens, the weedy var. frutescens, and the weedy var. crispa was 0.402 g (0.297 g - 0.721 g), 0.157 g (0.095 g - 0.307 g), and 0.120 g (0.091 g - 0.166 g), respectively (Table 3).
Among the 10 qualitative characteristics, for the color of leaf adaxial side (QL1), one accession of cultivated var. frutescens was light green, 17 accessions were green, and three accessions were dark green; 7 accessions of weedy var. frutescens were green and 7 accessions were dark green; and the accessions of weedy var. crispa showed green (one accession), dark green (3 accessions), green/purple (10 accessions), and purple (3 accessions).
For color of leaf abaxial side (QL2), the accessions of cultivated var. frutescens showed light green (4 accessions) and green (17 accessions); the accessions of weedy var. frutescens showed light green (3 accessions), green (8 accessions), and purple (3 accessions); and the accessions of weedy var. crispa showed light green (1 accession) and purple (16 accessions).
For number of leaf teeth (QL3), the accessions of cultivated var. frutescens showed few (1 accession), middle (13 accessions), and many (7 accessions) types; the accessions of weedy var. frutescens showed few (9 accessions) and middle (5 accessions) types; and the accessions of weedy var. crispa showed few (8 accessions), middle (7 accessions), and many (2 accessions) types.
For stem color (QL4), the accessions of cultivated var. frutescens showed light green (12 accessions), green (7 accessions), and purple (2 accessions); the accessions of weedy var. frutescens showed light green (5 accessions), green (4 accessions), and purple (5 accessions); and the accessions of weedy var. crispa showed only purple (17 accessions).
For flower color (QL5), the accessions of cultivated var. frutescens showed only white (21 accessions); the accessions of weedy var. frutescens showed white (11 accessions) and purple (3 accessions); and the accessions of weedy var. crispa showed white (1 accession), white/purple (3 accessions), and purple (13 accessions).
For plant fragrance (QL6), the cultivated var. frutescens showed frutescens (19 accessions) and other (2 accessions) botanical aromas; the weedy var. frutescens showed frutescens (7 accessions), crispa (2 accessions), and other (5 accessions) botanical aromas; and the weedy var. crispa showed frutescens (1 accession), crispa (13 accessions), and other (3 accessions) botanical aromas.
For leaf shape (QL7), the cultivated var. frutescens showed lanceolate (7 accessions) and long oval (14 accessions) types; the weedy var. frutescens showed lanceolate (4 accessions), heart (3 accessions), and long oval (7 accessions) types; and the weedy var. crispa showed lanceolate (10 accessions), long oval (4 accessions),and wrinkle (3 accessions) types.
For seed color (QL8), the accessions of cultivated var. frutescens showed dark brown (10 accession), brown (7 accessions), gray (1 accession), and white (3 accessions); the accessions of weedy var. frutescens showed dark brown (12 accessions) and brown (2 accessions); and the accessions of weedy var. crispa showed dark brown (12 accessions), brown (4 accessions), and mixed (1 accession).
For seed size (QL9), all accessions of cultivated var. frutescens showed large seeds (21 accessions), while all weedy var. frutescens (14 accessions) and var. crispa (17 accessions) showed small seeds.
For seed hardness (QL10), the accessions of cultivated var. frutescens showed only soft (21 accessions) type, the accessions of weedy var. frutescens showed hard (13 accessions) and soft (1 accession) types, and the accessions of weedy var. crispa had only hard (17 accessions) type (Table 3).
2. Morphological differences among accessions of the CWTP collected from South Korea
Results of correlation analysis among the morphological characteristics of the 52 accessions of the CWTP collected from South Korea are shown in Table 4.

Pearson correlation coefficient between one quantitative and 10 qualitative characteristics for 52 accessions of the CWTP.
In our study, many morphological characteristics among the accessions of the CWTP showed statistically significant positive or negative correlation coefficients, with significance levels of 0.05 and 0.01.
The results showed that, among all morphological characteristics, the combinations between QN1 and QL10 (0.848**); between QL1 and QL2 (0.838**), QL4 (0.736**), and QL5 (0.770**); and between QL2 and QL4 (0.746**) and QL5 (0.843**) had the highest positive correlation coefficients compared with the other combinations, with significance levels of 0.01. Also the combinations between QN1 and QL9 (− 0.838**) and between QL9 and QL10 (−0.961**) showed the highest negative correlation coefficients compared with the other combinations, with significance levels of 0.01.
In addition, the combinations between QL1 and QL6 (0.522**) and QL9 (0.590**); between QL2 and QL6 (0.557**) and QL9 (0.583**); between QL3 and QL10 (0.546**); between QL4 and QL5 (0.630**), QL6 (0.628**), and QL9 (0.573**); between QL5 and QL9 (0.606**); and between QL6 and QL9 (0.511**) showed comparatively higher positive correlation coefficients than the other combinations, with significance levels of 0.01.
Also the combinations between QN1 and QL1 (−0.553**), QL2 (−0.546**), and QL5 (−0.598**); between QL1 and QL10 (−0.577**); between QL2 and QL10 (−0.602**); between QL3 and QL9 (−0.540**); between QL4 and QL10 (−0.575**); between QL5 and QL10 (−0.631**); and QL9 and QL10 (−961**) showed comparatively higher negative correlation coefficients than the other combinations, with significance levels of 0.01. The remaining combinations showed lower positive or negative correlation coefficients, with significance levels of 0.01 and 0.05 (Table 4).
Meanwhile, with the PCA, the first and second principal components accounted for 51.7% and 13.5%, respectively, of the total variance (Table 5). For the first principal component in the PCA, one quantitative and most of the qualitative characteristics made a significant contribution in either the positive or negative direction of the first axis, such as QN1, QL1, QL2, QL4, QL5, QL9, and QL10 (Table 5).

Cumulative variances of first and second principal components and the loadings of one quantitative and 10 qualitative characteristics on each principal component.
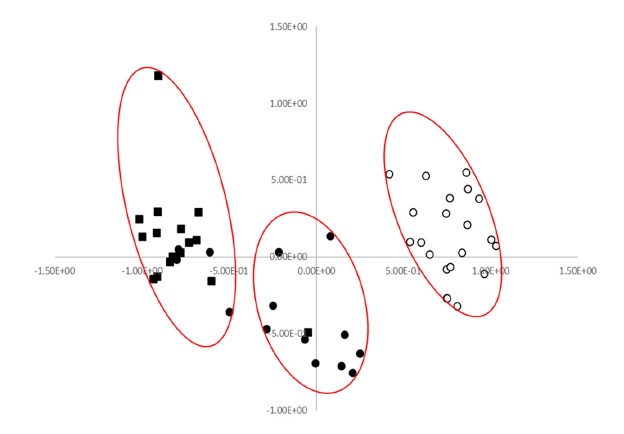
Based on the first axis of the PCA (Fig. 1), all accessions of cultivated var. frutescens were located on the right side, while most accessions of weedy var. frutescens were located on the left side.
In addition, all accessions of weedy var. crispa were located on the left side of the first axis. Therefore, all accessions of cultivated var. frutescens were clearly separated from the accessions of weedy var. crispa on the first axis. In addition, all accessions of cultivated var. frutescens were relatively clearly separated from the accessions of weedy var. frutescens, and most of the accessions of weedy var. frutescens and var. crispa were clearly separated on the first axis by the PCA (Fig. 1).
DISCUSSION
In crop species, differences in morphological characteristic changes between native landraces and wild species in accordance with geographic distribution will provide taxonomically important clues to understanding the evolutionary or domestication process of a specific crop (Gould and Johnston, 1972; Wyatt and Antonovics, 1981; Ohnishi, 2001; Ma and Lee, 2017; Lee and Sa et al., 2018).
In recent years, many taxonomic studies have been performed using morphological characteristics to distinguish CWTP accessions. Meanwhile, domestication is the evolutionary process from wild species to cultivated varieties, and domesticated crops are morphologically (such as seed size and plant size) and physiologically (such as seed dormancy and flowering time) different from their wild ancestors (Schwanitz, 1966; Hancock, 1992; Harlan, 1992; Ha et al., 2021).
Morphological characteristics are easily affected by environmental conditions; therefore, relying on phenotypic traits is an inaccurate method for understanding the genetic variation within crop species. However, as described in the Introduction, molecular markers have been applied successfully for identifying and classifying plant germplasm resources.
Nonetheless, the identification and description of phenotypic morphological traits are still the most basic methods of genetic variation analysis among accessions of crop germplasm resources and cannot be replaced completely by molecular marker technology (Liao et al., 2015).
Therefore, in this study, 52 accessions of Perilla and its weedy types collected from South Korea were used to investigate variation in morphological characteristics and classification of the CWTP.
In our study, one quantitative characteristic and 10 qualitative characteristics of 52 accessions of the CWTPC from South Korea were compared and analyzed as shown in Table 3.
For QN1, the accessions of cultivated var. frutescens showed significant differences compared with accessions of weedy var. frutescens and var. crispa. In recent years, Perilla seed oil, which contains various amino acids and has many health benefits, has attracted worldwide attention as a natural health care product.
Cultivated var. frutescens is widely cultivated and used in South Korea because it has much higher seed oil content than other oil crops. In particular, in East Asia the cultivated var. frutescensis considered as a highly domesticated crop by natural or human selection during the domestication process because the cultivated var. frutescens shows more variation in morphological characteristics such as seed size and weight compared with its weedy type (Lee and Ohnishi, 2003; Sa et al., 2013). Thus, the significantly higher seed weight of cultivated var. frutescens compared with that of the two weedy types of Perilla was considered to be because of strong selection by farmers.
Meanwhile, the cultivated var. crispa is not cultivated at all in South Korea today, while the weedy var. crispa grows naturally. However, in Japan, the cultivated var. crispa is cultivated as a leaf vegetable by following traditional methods in which seedlings of cultivated var. crispa that sprouted in the field from seed shattering during autumn of the previous year are transplanted into fields for cultivation.
In South Korea, cultivated var. frutescens is cultivated by sowing the seeds harvested in the previous fall in the field (Nitta et al., 2003; Sa et al., 2013; Ha et al., 2021). Therefore, the cultivation methods of the two cultivated types of Perilla in South Korea and Japan show a distinct difference.
The morphological differences between accessions of cultivated var. frutescens and weedy var. crispa (Table 3) were shown by the following qualitative characteristics: QL1, QL2, QL4, QL5, QL6, QL9, and QL10. The only significant differences between the cultivated and weedy types of var. frutescens were seed characteristics QL9 and QL10, and there were no significant morphological differences in the other remaining characteristics.
In addition, the morphological differences among the accessions of the two weedy types of var. frutescens and var. crispa were shown by QL1, QL2, and QL5. As suggested by Lee and Ohnishi (2001), the color of leaf adaxial side, color of leaf abaxial side, stem color, flower color, plant fragrance, seed size, and seed hardness were useful morphological characteristics for distinguishing between cultivated var. frutescens and cultivated var. crispa.
In particular, the cultivated var. frutescens and two weedy types of Perilla can be accurately distinguished based on the seed-related characteristics among these morphological characteristics. The different degrees of morphological difference among accessions of the CWTP collected from South Korea reflect the morphological diversity of the Korean Perilla germplasm accessions.
From the results of Pearson correlation coefficient analysis (Table 4), among the 11 morphological characteristics, the leaf characteristics showed the following correlation coefficients: the combinations between QL1 and QL2 (0.838**), QL4 (0.736**), and QL5 (0.770**) and between QL2 and QL4 (0.746**) and QL5 (0.843**) showed the most statistically significant positive correlation coefficients compared with the other combinations at p < 0.01.
Among the seed characteristics, the combinations between QN1 and QL9 (−0.838**) and QL10 (0.848**) and between QL9 and QL10 (−0.961**) showed the most statistically significant positive or negative correlation coefficients compared with the other combinations at p < 0.01.
In addition, the combinations between QL1 and QL6 (0.522**) and QL9 (0.590**); between QL2 and QL6 (0.557**) and QL9 (0.583**); between QL3 and QL10 (0.546**); between QL4 and QL5 (0.630**), QL6 (0.628**), and QL9 (0.573**); between QL5 and QL9 (0.606**); and between QL6 and QL9 (0.511**) showed statistically significant positive correlation coefficients. Also the combinations between QN1 and QL1 (−0.553**), QL2 (−0.546**), and QL5 (−0.598**); between QL1 and QL10 (−0.577**); between QL2 and QL10 (−0.602**); between QL3 and QL9 (-0.540**); between QL4 and QL10 (−0.575**); and between QL5 and QL10 (−0.631**) showed statistically significant negative correlation coefficients at a significance level of 0.01 (Table 4).
According to our current results, among the 11 morphological characteristics, most of the characteristics related to leaves and seeds showed statistically significant correlations among the accessions of the CWTP collected from South Korea. Therefore, traits related to leaves and seeds were found to be useful for distinguishing between accessions of the CWTP.
PCA can transform multiple variables into a few principal components. For further evaluation using the 11 morphological characteristics among the 52 accessions of the CWTP collected from South Korea, PCA was conducted (Table 5). Among all the principal components, the first and second principal components accounted for 51.7% and 13.5%, respectively, of the total variance (Table 5).
As the first principal component in the PCA, QN1, QL1, QL2, QL4, QL5, QL9, and QL10 contributed significantly to the positive or negative direction of the first axis (Table 5).
Therefore, as reported by Lee and Ohnishi (2001), these characteristics are considered useful characteristics for distinguishing between accessions of the CWTP.
In particular, on the first axis of the PCA, the accessions of cultivated var. frutescens and the two weedy types of Perilla collected from South Korea were relatively clearly separated. Also, most accessions of weedy var. frutescens and var. crispa were relatively clearly separated from each other by the first axis (Fig. 1).
The accessions of weedy var. frutescens are between the accessions of cultivated var. frutescens and weedy var. crispa on the first axis. Although a clear distinction was found by the PCA between accessions of cultivated and weedy types of Perilla, it was difficult to distinguish them clearly.
In recent years, there have been many studies of classification between var. frutescens and var. crispa. As reported in the Introduction, because there is an intermediate type between var. frutescens and var. crispa, it is impossible to distinguish fully between the two varieties of Perilla (Honda et al., 1990; Lee and Ohnishi, 2001, 2003). The two cultivated types of var. frutescens and var. crispa can be crossed by artificial pollination (Nagai, 1935; Honda et al., 1990; Honda et al., 1994; Lim et al., 2019; Kim et al., 2021; Lim et al., 2021).
In this study, there were a few special accessions of weedy var. frutescens similar to cultivated var. frutescens or weedy var. crispa. These accessions of weedy var. frutescens may be derived from escape forms from accessions of cultivated var. frutescens, or they may be hybrids between CWTP accessions (Nitta and Ohnishi, 1999; Lee et al., 2002; Lee and Ohnishi, 2003; Lee and Kim, 2007; Sa et al., 2013).
The morphological characteristics of the CWTP are highly variable and easily affected by environmental conditions; therefore, future taxonomic studies of Perilla species should be performed by combining molecular marker techniques and morphological characteristics.
This study provides useful information for future evaluation and classification of the CWTP collected from South Korea. Furthermore, based on morphological characteristics, we have observed that it is difficult to distinguish completely between the cultivated and weedy types of var. frutescens, as well as between the two weedy types of var. frutescens and var. crispa. Morphological characteristics can reflect the genetic variation of crop species.
In this study, by investigating the morphological characteristics of Perilla accessions collected from South Korea, we revealed the diversity of morphological characteristics among accessions of the CWTP. Therefore, the results of this study are expected to provide useful information for understanding the morphological variation of the CWTP genetic resources collected in Korea.
Acknowledgments
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1F1A1063300) and the Cooperative Research Program for Agriculture Science and Technology Development (PJ014227032019 and PJ0142272019), Rural Development Administration, Korea.
References
-
Asif M. (2011). Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Oriental Pharmacy and Experimental Medicine. 11:51-59.
[https://doi.org/10.1007/s13596-011-0002-x]

-
Fu ZY, Sa KJ, Park H, Jang SJ, Kim YJ and Lee JK. (2022). Utilization of novel Perilla SSR markers to assess the genetic diversity of native Perilla germplasm accessions collected from South Korea. Plants. 11:2974. https://www.mdpi.com/2223-7747/11/21/2974, (cited by 2022 Oct 31).
[https://doi.org/10.3390/plants11212974]

-
Gould SJ and Johnston RF. (1972). Geographic variation. Annual Review of Ecology, Evolution, and Systematics. 3:457-498.
[https://doi.org/10.1146/annurev.es.03.110172.002325]

-
Ha YJ, Sa KJ and Lee JK. (2021). Identifying SSR markers associated with seed characteristics in Perilla(Perilla frutescens L.). Physiology and Molecular Biology of Plants. 27:93-105.
[https://doi.org/10.1007/s12298-021-00933-3]

- Hancock JF. (1992). Plant evolution and the origin of crop species. CABI Publishing. Cambridge. MA, USA. p.99-130.
- Harlan JR. (1992). Origins and processes of domestication. In Chapman GP. (ed.). Grass evolution and domestication. Cambridge University Press. Cambridge, England. p.159-175.
-
Hashimoto M, Matsuzaki K, Kato S, Hossain S, Ohno M and Shido O. (2020). Twelve-month studies on Perilla oil intake in Japanese adults-possible supplement for mental health. Foods. 9:530. https://www.mdpi.com/2304-8158/9/4/530, (cited by 2022 October 31).
[https://doi.org/10.3390/foods9040530]

-
Honda G, Koezuka Y and Tabata M. (1990). Genetic studies of fruit color and hardness in Perilla frutescens. Japanese Journal of Breeding. 40:469-474.
[https://doi.org/10.1270/jsbbs1951.40.469]

- Honda G, Yuba A, Kojima T and Tabata M. (1994). Chemotaxonomic and cytogenetic studies on Perilla frutescens var. citiodora(‘Lemon Egoma’). Nature Medicine. 48:185-190.
-
Kim JY, Sa KJ, Ha YJ and Lee JK. (2021). Genetic variation and association mapping in F2 population of Perilla crop (Perilla frutescens L.) using new developed Perilla SSR markers. Euphytica. 217:135. https://link.springer.com/article/10.1007/s10681-021-02867-z, (cited by 2021 June 4).
[https://doi.org/10.1007/s10681-021-02867-z]

- Lee JK and Kim NS. (2007). Genetic diversity and relationships of cultivated and weedy types of Perilla frutescens collected from East Asia revealed by microsatellite markers. Korean Journal of Breeding Science. 39:491-499.
-
Lee JK and Ohnishi O. (2001). Geographical differentiation of morphological characters among Perillacrops and their weedy types in East Asia. Breeding Science. 51:247-255.
[https://doi.org/10.1270/jsbbs.51.247]

- Lee JK and Ohnishi O. (2003). Genetic relationships among cultivated types of Perilla frutescensand their weedy types in East Asia revealed by AFLP markers. Genetic Resources and Crop Evolution. 50:65-74.
-
Lee JK, Nitta M, Kim NS, Park CH, Yoon KM, Shin YB and Ohnishi O. (2002). Genetic diversity of Perilla and related weedy types in Korea determined by AFLP analyses. Crop Science. 42:2161-2166.
[https://doi.org/10.2135/cropsci2002.2161]

-
Li HL. (1969). The vegetables of ancient China. Economic Botany. 23:235-260.
[https://doi.org/10.1007/BF02860457]

- Liao L, Chen YH, Zhao YR, Wang XL, Bai CJ and Wang ZY. (2015). Morphology diversity of Axonopus compressus germplasm. Pratacultural Science. 2:248-257.
-
Lim SE, Sa KJ and Lee JK. (2021). Bulk segregant analysis identifies SSR markers associated with leaf-and seed related traits in Perilla crop(Perilla frutescens L.). Genes and Genomics. 43:323-332.
[https://doi.org/10.1007/s13258-021-01056-5]

-
Lim SE, Sa KJ, Ha YJ and Lee JK. (2019). Genetic analysis of F2 population derived from the cross between Perilla frutescens var. frutescens and var. crispa. Korean Journal of Breeding Science. 51:184-189.
[https://doi.org/10.9787/KJBS.2019.51.3.184]

-
Ma SJ and Lee JK. (2017). Morphological variation of two cultivated types ofPerillacrop from different areas of China. Horticultural Science and Technology. 35:510-522.
[https://doi.org/10.12972/kjhst.20170054]

-
Ma SJ, Sa KJ, Hong TK and Lee JK. (2017). Genetic diversity and population structure analysis in Perilla frutescens from Northern areas of China based on simple sequence repeats. Genetics and Molecular Research. 16:16039746. https://link.springer.com/article/10.1007/s13258-018-0756-3, (cited by 2021 Sep 21).
[https://doi.org/10.4238/gmr16039746]

-
Ma SJ, Sa KJ, Hong TK and Lee JK. (2019). Genetic diversity and population structure analysis in Perilla crop and their weedy types from northern and southern areas of China based on simple sequence repeat(SSRs). Genes and Genomics. 41:267-281.
[https://doi.org/10.1007/s13258-018-0756-3]

- Makino T. (1961). Makino’s new illustrated flora of Japan. Hokuryukan Co. Tokyo, Japan. 1060 p.
-
Nitta M and Ohnishi O. (1999). Genetic relationships among two Perilla crop, shiso and egoma, and the weedy type revealed by RAPD markers. Genes and Genetic Systems. 74:43-48.
[https://doi.org/10.1266/ggs.74.43]

-
Nitta M, Lee JK and Ohnishi O. (2003). Asian Perilla crop and their weedy forms: Their cultivation, utilization and genetic relationships. Economic Botany. 57:245-253.
[https://doi.org/10.1663/0013-0001(2003)057[0245:APCATW]2.0.CO;2]

-
Nitta M, Lee JK, Kang CW, Katsuta M, Yasumoto S, Liu D, Nagamine T and Ohnishi O. (2005). The distribution of Perilla species. Genetic Resources and Crop Evolution. 52:797-804.
[https://doi.org/10.1007/s10722-003-6017-5]

- Nitta M. (2001). Origin Perilla crops and their weedy type. Ph.D. Thesis. Kyoto University. Kyoto, Japan. p.78.
-
Park H, Sa KJ, Hyun DY, Lee S and Lee JK. (2021). Identifying ssr markers related to seed fatty acid content in Perilla crop(Perilla frutescens L.). Plants. 10:1404. https://www.mdpi.com/2223-7747/10/7/1404, (cited by 2021 July 9).
[https://doi.org/10.3390/plants10071404]

-
Park H, Sa KJ, Lee SK and Lee JK. (2022). Genetic variation of seed oil characteristics in native Korean germplasm of Perilla crop(Perilla frutescens L.) using SSR markers. Genes and Genomics. 44:1159-1170.
[https://doi.org/10.1007/s13258-022-01289-y]

-
Park YJ, Lee JK and Kim NS. (2009). Simple sequence repeat polymorphisms(SSRPs) for evaluation of molecular diversity and germplasm classification of minor crops. Molecules. 14:4546-4569. https://www.mdpi.com/1420-3049/14/11/4546, (cited by 2021 November 10).
[https://doi.org/10.3390/molecules14114546]

- Rohlf FJ. (1998). NTSYS-pc: Numerical taxonomy and multivariate analysis system version 2.0 user guide. Exter Software. Setauket. NY, USA. p.6-48.
-
Sa KJ, Choi IK, Park KC and Lee JK. (2018). Genetic diversity and population structure among accessions of Perilla frutescens (L.) Britton in East Asia using new developed microsatellite markers. Genes and Genomics. 40:1319-1329.
[https://doi.org/10.1007/s13258-018-0727-8]

-
Sa KJ, Choi SH, Ueno M and Lee JK. (2015). Genetic diversity and population structure in cultivated and weedy types of Perilla in East Asia and other countries as revealed by SSR markers. Horticulture, Environment, and Biotechnology. 56:524-534.
[https://doi.org/10.1007/s13580-015-0039-8]

-
Sa KJ, Choi SH, Ueno M, Park KC, Park YJ, Ma KH and Lee JK. (2013). Identification of genetic variations of cultivated and weedy types of Perilla species in Korea and Japan using morphological and SSR markers. Genes and Genomics. 35:649-659.
[https://doi.org/10.1007/s13258-013-0117-1]

-
Sa KJ, Kim DM, Oh JS, Park H, Hyun DY, Lee S, Rhee JH and Lee JK. (2021). Construction of a core collection of native Perilla germplasm collected from South Korea based on SSR markers and morphological characteristics. Scientific Reports. 11:23891. https://www.nature.com/articles/s41598-021-03362-0, (cited by 2021 December 13).
[https://doi.org/10.1038/s41598-021-03362-0]

- Schwanitz F. (1966). The origin of cultivated plants. Harvard University Press. Cambridge. MA, USA. p.175.
-
Wyatt R and Antonovics J. (1981). Butterfly weed re-revisited: Spatial and temporal patterns of leaf shape variation inAsclepias tuberosa. Evolution. 35:529-542.
[https://doi.org/10.1111/j.1558-5646.1981.tb04915.x]

- Yamane Y. (1950). Cytogenetic studies in Perilla and Coleus. I. Chromosome numbers. The Japanese Journal of Genetics. 25:220-220.