
Protective Effect of Polysaccharide Derived from Natural Extract on Human Follicle Dermal Papilla Cell and Keratinocytes
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study sought to evaluate the skin-protective effects of polysaccharide extracts derived from four natural materials (ginseng, green tea, shiitake, and aloe vera) to determine their potential as raw materials for the cosmetics industry. In a broad sense, we aimed to determine the effects of skin protection on preventing hair loss.
Polysaccharide extracts were prepared from ginseng, green tea, shiitake, and aloe vera. For each polysaccharide extract, the total sugar content was assessed, and antioxidant activity was measured using superoxide dismutase-like activity assay. The efficacy of the extracts at enhancing cell proliferation and hydrogen peroxide-induced apoptosis were assessed in human follicle dermal papilla cells (HFDPC) and keratinocyte (HaCaT) cells.
Each of the tested polysaccharides effectively protected dermal papilla cells and keratinocytes by promoting cell proliferation and/or suppressing apoptosis. The protective effect on hair-related keratinocytes and dermal papilla cells is expected to have a positive effect on the prevention of hair loss. Therefore, we propose that ginseng, green tea, shiitake, and aloe vera polysaccharide extracts each show promise to be developed as effective pharmaceutical and/or cosmetic ingredients for preventing hair loss.
Keywords:
Panax ginseng, Aloe Vera, Green Tea, Human Follicle Dermal Papilla Cells, Keratinocytes, Polysaccharides, Shiitake MushroomINTRODUCTION
Modern people have increased their economic development and standard of living, their desire for external beauty has increased, and they have pursued not only aesthetic beauty but also internal beauty and health (Kim et al., 2018).
Person might express their individuality and beauty through various changes in hairstyle, such as dyeing and perming, but these processes can cause hair and scalp damage, and in the longer term may be associated with hair loss. Additional factors are constantly increasing the hair-loss population among modern people, including aging, genetic factors, industrial pollution, stress, overdose of ready-to-eat foods, mineral deficiency, and abuse of chemical products (Choi et al., 2021).
Hair grows in hair follicles via a four-stage hair-growth cycle (anagen, catagen, telogen, exogen), and the hair of a given follicle exhibits an oscillation between growth and shedding (Park et al., 2016).
Hair loss is a process wherein the hair on the head and body becomes thinner and decreases due to the shrinkage and reduction of hair follicles during the growth period. Simply put, hair loss is a condition in which, due to various causes, there is no hair in an area where it should normally exist.
Hair loss is known to be controlled by factors such as inhibition or functional decline of human hair follicle dermal papilla cells, decreased blood flow to the scalp, and abnormality of the hair-growth cycle due to the action of androgens, psychological stress, and/or environmental pollution. Hair loss is divided into "natural hair loss", which occurs during the telogen phase of the hair-growth cycle, and "abnormal hair loss", which occurs during the caused by environmental factors, such as stress and illness (Lee et al., 2009; Moreno et al., 2017).
Scalp and hair care products made from natural products may be less effective, but they offer the advantage of being safer than chemically synthesized single substances (Park et al., 2011).
Polysaccharides show various physiological effects. For example, glycosaminoglycans (GAGs), a class of polysaccharides, and their analogues exhibit diverse biological activities and can be used to modulate vascular cell proliferation (Chupa et al., 2000). A polysaccharide isolated from the traditional Chinese herb Bletilla striata (Thunb.) was reported to induce human umbilical vascular endothelial cell proliferation and vascular endothelial growth factor (VEFG) expression in vitro (Wang et al., 2006). In addition, Hottuynia cordata extract, a traditional Chinese medicinal plant widely used as an ingredient in complex formulations for the treatment of alopecia in Asia, has recently been reported as an important source of natural polysaccharides and flavonoids. Hottuynia cordata extract could support hair growth by stimulating proliferation of DPCs and elongating anagen stage (Kim et al., 2019).
Among the natural products, ginseng has been studied extensively for its anti-aging (Hwang et al., 2017) and whitening (Kim et al., 2015) effects. Moreover, herb extracts from cultured Korean wild ginseng have been shown to increase the density and thickness of hair by preventing hair loss and promoting hair growth (An et al., 2009).
Green tea has been reported to have beneficial effects on skin, such as photoprotection (Lim et al., 2014), antioxidation (Farooq et al., 2018), and whitening (Song et al., 2015).
Shiitake mushroom, which is named for its flower-like shape and the white tortoise shell-like pattern made by its cracked upper surface, has an antioxidant effect on skin (Cheung et al., 2003) as well as antibacterial activity (Kitzberger et al., 2007).
Aloe vera has been reported to enhance immunity (Im et al., 2016) and have moisturizing (Dal'Belo et al., 2006), antioxidant (Hu et al., 2003), and anti-inflammatory (Vijayalakshmi et al., 2012) effects.
As mentioned above, the skin-benefiting effects of ginseng, green tea, shiitake mushrooms and aloe vera extracts as natural materials have been continuously studied. However, the skin effects of these polysaccharides and complexes have been little studied and reported. In particular, much less is known about the scalp protection and hair loss.
For this reason, we elucidated the protective effects of four polysaccharide extracts on HFDPC and keratinocytes in the current study.
Here, we generated polysaccharide extracts of ginseng, green tea, aloe vera, and shiitake. For each polysaccharide extract, we assessed the total sugar content, evaluated the ability to promote cell proliferation and protect against H2O2-induced apoptosis in HFDPC and HaCaT cells, and examined the antioxidant efficacy using a SOD-like activity analysis.
MATERIAL AND METHOD
1. Preparation of four kinds of polysaccharide extracts
Ginseng (Panax ginseng) roots cultivated Jeollanam-do Jangseong-gun, Korea, was crushed, mixed with 20 volumes of water, and extracted at 80℃ for 6 hours. The resulting extract was collected by centrifugation, filtered through filter paper (AF-31H, HM, Seoul, Korea), and then concentrated to 1/5 in a rotary vacuum concentrator (EYELA N-2100, Tokyo Rikakikai Co., Tokyo, Japan). Three volumes of ethanol was slowly added to the concentrate; when the resulting precipitate sank, the supernatant was discarded. This ethanol washing step was repeated two more times. The final polysaccharide precipitate was recovered with filter paper (HM, Seoul, Korea) and dried with a dryer.
The above process was also carried out for green tea and shiitake cultivated in Jeju to produce polysaccharide powder. To obtain aloe vera polysaccharide, 20 volumes of water was added to dried leaves of Jeju aloe vera and extraction was performed for 3 hours. Centrifugation was used to obtain the extract, activated carbon (DX-Ultra, Norit Americas Inc., Marshall, TX, USA) was stirred in to deodorize and decolorize the extract, and the extract was filtered (HU-040, AF-31H, UH-005; HM, Seoul, Korea).
Aloe vera polysaccharide was precipitated by adding 3.2 volumes of ethanol, after that immersion for 24 hours, the precipitated polysaccharides were obtained, dried in a dryer, and then pulverized to produce aloe vera polysaccharide powder. The four polysaccharide powders were dissolved in DMSO for the cell-based experiments.
2. Evaluation of total sugar content analysis
Total sugar content was measured using the phenol-sulfate method.
Briefly, 1 ㎖ of 5% phenol solution and 5 ㎖ of sulfuric acid were sequentially mixed with 1 ㎖ of sample extract, and the reaction was allowed to proceed at room temperature for 30 minutes.
Absorbance was measured at 485 ㎚ using a Microplate reader (Multiskan GO, Thermo Fisher Scientific, Waltham, MA, USA). As a control, 1 ㎖ of water was processed as described for the sample.
The total sugar content was calculated using a standard quantitative curve derived from a glucose standard (Sigma-Aldrich Co., St. Louis, MO, USA).
3. Cell culture and evaluation of cell viability
Human cells representing dermal keratinocytes (HaCaT cells), which are the main cells that form hair roots, and primary human follicle dermal papilla cells (HFDPC), which regulate the activity of hair root-forming cells, were used.
HaCaT cells were kindly provided by the Amorepacific Corporation R&D Center (Yongin, Korea) and grown in Dulbecco’s modified Eagle’s medium (DMEM; Welgene Inc., Gyeongsan, Korea) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 1% penicillin-streptomycin (Lonza, Basel, Switzerland), at 37℃ in an atmosphere containing 5% CO2. The cells were cultured in T75 cell culture flasks (SPL Life Science, Pocheon, Korea). The HFDPC was purchased from Promocell (Heidelberg, Germany) and cultured in Follicle Dermal Papilla Cell Growth Medium (C-26501, Promocell, Heidelberg, Germany) and Supplement Mix (C-39625, Promocell, Heidelberg, Germany) in a T75 cell culture flask (SPL Life Science, Pocheon, Korea) at 37℃ in an atmosphere containing 5% CO2. When cell density at least 80% in the flask, cell subculture was performed.
Cell viability was determined using the WST-1 assay (EZ-Cytox, DoGenBio, Seoul, Korea) according to the manufacturer’s instructions.
Briefly, cells were seeded in 96-well plates and cultured for 24 hours. Test samples were added at different concentrations, the cells were incubated for 24 hours, 100 ㎕ of medium containing 10 ㎕ of WST (water-soluble tetrazolium salt) solution was added to each well, and the plates were incubated for 1 hour at 37℃.
The absorbance of each well at 450 nm was measured using a Microplate Reader (Multiskan GO, Thermo Fisher Scientific, MA, USA). Cell viability was calculated using the formula:
4. Evaluation of anti-hair loss effect
HFDPC and HaCaT cells were dispensed to 96-well plates at 0.3 × 104 cells in 100 ㎕/well and cultured for 1 day. For the assay of HFDPC cell proliferation, the control group was treated with medium containing 20% s upplement, and the positive control group was treated with medium containing 100% supplement.
For the assay of HaCaT cell proliferation, the control group was treated with medium containing 2% FBS and the positive control group was treated with medium containing 10% FBS. For the treatment groups, each sample was applied in medium containing 20% s upplement (HFDPC) or 2% FBS (HaCaT).
The cells were cultured for 72 hours, the supernatant was removed, and WST-1 reagent diluted to 10% was added to each well. After the plates were incubated at 37℃ in 5% CO2 for 1 hour, absorbance was measured at 450 nm using a microplate reader (Multiskan GO, Thermo Fisher Scientific Inc., Waltham, MA, USA).
First, the concentration range of hydrogen peroxide (H2O2, a typical active oxygen) that induced oxidative stress-induced cell death was determined in preliminary tests.
Next, we found that the concentrations of H2O2 inducing cell death were about 400 μM for HFDPC and 1,000 μM for HaCaT cells. In the main tests, cells (0.6 × 104 HFDPC/well or 1.0 × 104 HaCaT cells/well in 100 ㎕) were dispensed to 96-well plates and cultured for 1 day.
Next, we treated the indicated concentrations of polysaccharide extracts for 1 day in H2O2-treated cells. After that the supernatant was removed, and WST-1 reagent was added to the medium at a final concentration of 10%. The cells were incubated for 1 hour at 37℃ in 5% CO2, and absorbance was measured at 450 ㎚ using a microplate reader.
5. Evaluation of anti-oxidant effect
The SOD-like activity of each sample was measured using an EZ-SOD assay kit (DogenBio, Seoul, Korea).
Test samples were added at different concentrations (20 ㎕), and was mixed with the water-soluble tetrazolium salt (WST) working solution (200 ㎕) and reacted with the enzyme working solution at 37℃ for 20 minutes.
Absorbance was measured at 450 ㎚ using a microplate reader. The SOD-like activity of each test sample was normalized to the corresponding control value, as follows:
A, absorbance of the experimental (extract-added).
B, absorbance of the extract-free control group.
Both A and B are the values excluding the absorbance of the control group.
6. Statistical analysis
All analytical values were obtained by repeating 3 times and expressed as mean and standard deviation (Means ± SD). Comparisons between samples were statistically processed based on Student's t-test via the Microsoft Office (Raymond, WA, USA) Excel T-TEST function and determined to be significant if the p value was less than 0.05.
RESULTS AND DISCUSSION
1. Measurement of total sugar content of four polysaccharides
In this experiment, the total sugar content of each polysaccharide extract from each sample was measured, and the results are shown in Table 1.

Total sugar content analysis by phenol-sulfuric acid method for ginseng, shiitake, green tea, and aloe vera polysaccharides.
The sugar content of the shiitake polysaccharide extract is 560.55 ㎎/g, which is the highest content. And is 504.34 ㎎/g and 275.74 ㎎/g, 144.81 ㎎/g. respectively, in the order of ginseng polysaccharide extract, green tea polysaccharide extract and aloe vera polysaccharide extract.
2. Cytotoxicity assessment of HaCaT and HFDPC for four polysaccharides
Before assessing the cell protective effects of ginseng, green tea, shiitake, and aloe vera polysaccharide extracts in HFDPC and HaCaT cells, we used the WST reagent to identify the cytotoxic concentration for each polysaccharide extract (Fig. 1).
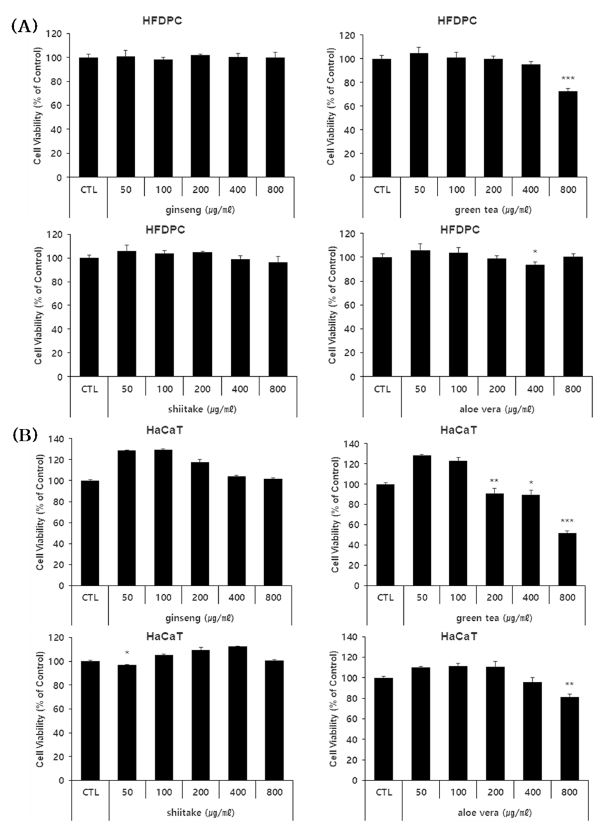
Cell viability of HFDPC and HaCaT cells treated with various concentrations of the four polysaccharide extracts.(A) Viability of HFDPC after exposure to the indicated doses of the four polysaccharides. (B) Viability of HaCaT cells after exposure to the indicated doses of the four polysaccharides. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to the control (CTL).
In HFDPC tested with 50 to 800 ㎍/㎖, survival of 90% or more was seen even at 800 ㎍/㎖ for ginseng, shiitake, and aloe vera polysaccharide extracts, indicating that these extracts were not toxic.
For the green tea polysaccharide extract, the survival rate of HFDPC was 70% at 800 ㎍/㎖, and no cytotoxicity was observed with concentrations of 400 ㎍/㎖ or less (Fig. 1A).
In HaCaT cells tested with 50 to 800 ㎍/㎖ of each extract, those treated with aloe vera and green tea polysaccharide extracts showed survival of 90% or more at concentrations at or below 400 ㎍/㎖ and 200 ㎍/㎖, respectively. HaCaT cells treated with shiitake or ginseng polysaccharide extracts exhibited survival of 90% or more even at 800 ㎍/㎖, indicating that there was no toxicity (Fig. 1B).
Based on these results, we used sub-toxic concentrations of each polysaccharide extract in HFDPC and HaCaT cells for our subsequent experiments.
3. Effect of four polysaccharides on cell proliferation in HFDPC cells
Samples of ginseng, green tea, shiitake, and aloe vera polysaccharide extract were applied to HFDPC at sub-toxic concentrations, and cell viability was assessed at day 3 post-treatment.
Significant increases in cell proliferation were seen in HFDPC treated with ginseng (all tested concentrations), aloe vera (at the lowest tested concentration), and green tea (at two of the lower tested concentrations) polysaccharide extracts (Fig. 2A, B and D). But shiitake was excluded because it was very lower significance and only one tested concentration significant result (Fig. 2C).
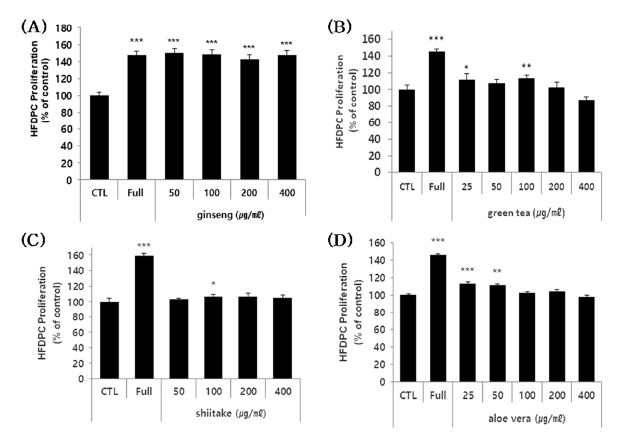
Effects of the polysaccharides on HFDPC proliferation.(A - D) HFDPC were treated with the indicated concentrations of the four polysaccharides for 72 hours, and cell proliferation was estimated using a colorimetric WST-1 assay kit. The results are shown as the percentage compared to control group (Means ± SD). Full (positive control); 100% supplement (necessary nutrient basic condition of cell growth) * p < 0.05, ** p < 0.01, *** p < 0.001 compared to the control (CTL).
The level of cell proliferation enhancement ranged from 10 to 50% depending on the type and concentration of the test sample, although some of the high concentration was not found to be cytotoxic after 24 h, it may have some cytotoxic effects under a longer culture time. These results indicate that the ginseng, green tea, and aloe vera polysaccharide extracts induce cell proliferation in a human follicle dermal papilla cells, indicating that they may have potential as materials for alleviating hair loss.
4. Effect of H2O2-induced apoptosis suppression in HFDPC cells of four polysaccharides
To assess the ability of each sample to suppress the H2O2 (oxidative stress)-induced cell death of HFDPC, we measured the viability of oxidatively stressed cells treated with sub-toxic doses of each extract.
Our results revealed that shiitake, aloe vera, and green tea polysaccharide extracts significantly recovered the viability of H2O2-treated HFDPC by 5.3% - 21.9% depending on the extract and applied concentration (Fig. 3). These results indicate that green tea, shiitake and aloe vera polysaccharide extracts can suppress the apoptosis of H2O2-treated HFDPC, and thus may have the potential to be used as hair loss-alleviating materials
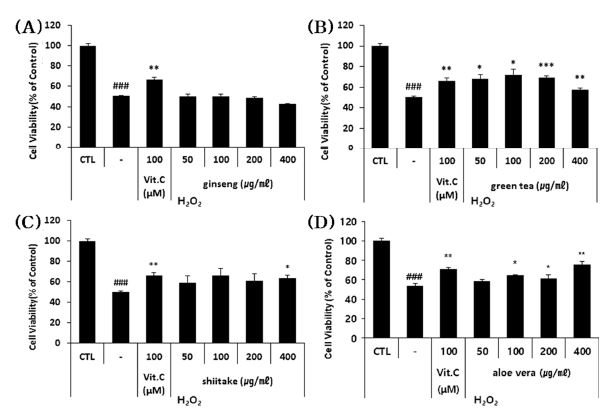
The effects of the four polysaccharide extracts on the H2O2-induced apoptosis of HFDPC.(A - D) The HFDPC were treated with the indicated concentrations of the four polysaccharides and H2O2 (400 μM) for 24 hour, and the number of cells recovered from H2O2 damage were estimated using a colorimetric WST-1 assay kit. The results are shown as the percentage compared to control group (Means ± SD). CTL; untreated group. -: cells treated with H2O2 alone (stimulator). Positive control; Vit.C (Vitamin C). * p < 0.05, ** p < 0.01, *** p < 0.001 compared to cells treated with H2O2 alone.
5. Effect of four polysaccharides on cell proliferation in HaCaT cells
To assess whether the polysaccharide extracts could potentially alleviate hair loss by acting on the keratinocytes that surround HFDPC, we examined their effects on HaCaT cells.
The cells were treated with various sub-toxic doses of each extract for 3 days, and cell viability was evaluated. Notably, the ginseng and shiitake polysaccharide extracts exhibited concentration-dependent cell proliferation-enhancing effects (Fig. 4A and C). For the green tea and aloe vera polysaccharide extracts, the lower tested doses yielded enhancement of cell proliferation (Fig. 4B and D). The extracts enhanced cell proliferation by 10% - 35%, depending on the extract and concentration tested. However, in the case of green tea and aloe vera polysaccharide, although the high concentration was not found to be cytotoxic after 24 hours, it has shown mild cytotoxic effects under a longer culture time (3 day).
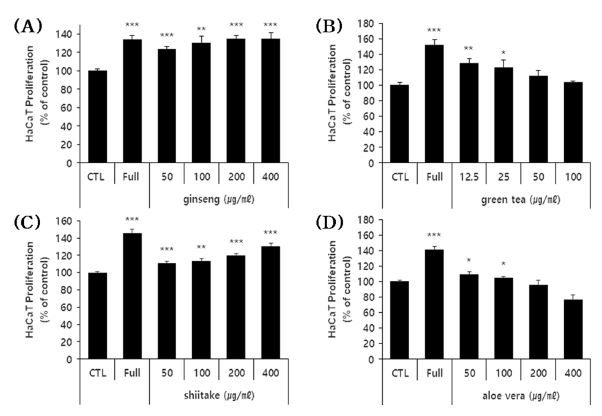
Effects of the four polysaccharide extracts on the proliferation of HaCaT cells.(A - D) HaCaT cells were treated with the indicated concentrations of the four polysaccharide extracts for 72 hours, and cell proliferation was estimated using a colorimetric WST-1 assay kit. The results are shown as the percentage compared to control group (Mean ± SD). (positive control); FBS 10%. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to the control (CTL).
These results indicate that all four polysaccharides could enhance the proliferation of HaCaT cells, and thus could potentially be useful as hair loss-alleviating materials.
6. Effect of H2O2-induced apoptosis suppression in HaCaT cells of four polysaccharides
To assess the ability of each polysaccharide extract to protect HaCaT cells against H2O2-induced apoptosis, we co-treated cells with H2O2 and sub-toxic doses of each extract and assessed cell viability after 24 hours. All four polysaccharides showed significant apoptosis-suppressing effects (Fig. 5). This suppression ranged from 2 to 50% depending on the extract and concentration.
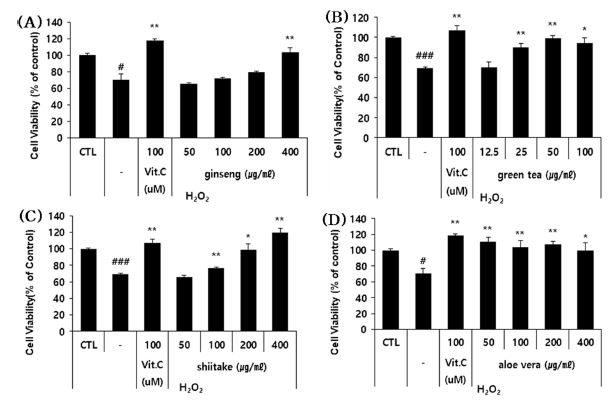
The effects of the four polysaccharides on the apoptosis of H2O2-treated HaCaT cells.(A - D) HaCaT cells were co-treated with the indicated concentrations of the four polysaccharides and H2O2 (1,000 μM) for 24 h, and cell viability was estimated using a colorimetric WST-1 assay kit. The results are shown as a percentage (Means ± SD) compared to the control group, which was taken as 100%. Control group: untreated group. -; stimulator (H2O2). Positive control: Vit. C (Vitamin C). * p < 0.05, ** p < 0.01, *** p < 0.001 compared to the stimulator (H2O2).
These results indicate all four polysaccharide extracts can suppress the H2O2-induced apoptosis of HaCaT cells and thus could have the potential to act as hair loss-alleviating materials.
7. SOD-like activity antioxidant effect of four kinds of polysaccharide extracts
SOD catalyzes the conversion of highly reactive superoxide anions into relatively less reactive hydrogen peroxide with an antioxidant defense enzyme in vivo, and can suppress aging effects and various diseases induced by active oxygen in the body (Pryor, 1986; Greenstock, 1993).
Natural SOD levels gradually decline with age, predisposing older individuals to oxidative stress-related disorders. In the context of skin, SOD reduces free radical damage; prevents wrinkles, fine wrinkles, and age spot; facilitates wound healing; and softens the skin. It is thus an important anti-aging ingredient and antioxidant in cosmetics and personal care products (Younus, 2018).
Here, we measured the SOD-like activity of the ginseng, green tea, shiitake and aloe vera polysaccharide extracts at various concentrations (Fig. 6). Three of the polysaccharides (excluding ginseng) exhibited significant SOD-like activity: At concentrations of 2 ㎎/㎖, the polysaccharide extract of green tea had SOD-like activity of 95.26%, that of aloe vera had activity of 88.58%, and that of shiitake had activity of 52.48%.
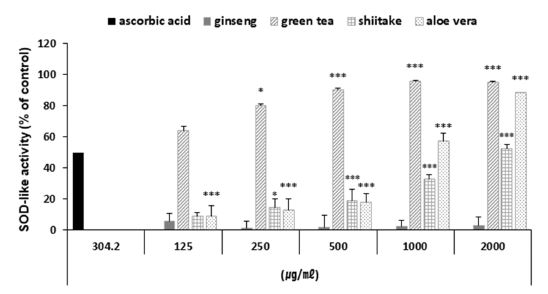
SOD-like activity of the four polysaccharide extracts.Values represent the means ± SD of three independent measurements. Positive; ascorbic acid. (* p < 0.05, ** p < 0.01, *** p < 0.001 compared to control).
Thus, the tested polysaccharide extracts could have SOD-like activity rates similar to that of SOD. These results indicate that three of the tested polysaccharides could be used as antioxidants in cosmetic materials.
The ultimate solution to hair loss treatment is to resolve the cause of hair loss. Numerous studies have been conducted to resolve hair loss by inhibiting the synthesis of the dihydrotestosterone (DHT) produced by 5-α reductase, which is a typical cause of hair loss.
Two commonly used drugs that suppress DHT synthesis are minoxidil and finasteride (Gupta et al., 2022; Chandrashekar et al., 2015); they effectively treat hair loss by inhibiting 5-α reductase activity, but can be associated with side effects such as erythema and itching. Therefore, researchers are continuing to search for effective hair loss-ameliorating substances that are derived from natural products and therefore are likely to have few side effects (Jung et al., 2019).
As modern people's interest in appearance is steadily increasing and hair loss is occurring not only in middle-aged and older generations but also in younger generations.
Here, we report evidence suggesting that polysaccharide extracts of ginseng, green tea, shiitake and aloe vera may have hair loss-relieving effects, although further investigation would be needed to define the component analysis and underlying mechanisms of anti-hair loss and the complex's hair loss effects.
Particularly, since these polysaccharide extracts contain other components besides the sugar component, it is thought that efficacy studies according to the components should be conducted in the future.
Collectively, the current study suggest that ginseng, green tea, shiitake and aloe vera polysaccharide extracts could be potent materials for hair loss protection through the proliferation of dermal papilla cells and keratinocytes, and inhibition of oxidative stress-induced apoptosis.
Additionally, these polysaccharides could be safe and effective ingredients for the development of anti-hair loss products in the growing hair loss industry and product market.
References
- An GS and Hwang IC. (2009). Hair loss prevention and hair growth promotion by herb extracts which contain the cultured Korean wild ginseng. Journal of Korean Society of Beauty and Art. 10:221-226.
-
Chandrashekar BS, Nandhini T, Vasanth V, Sriram R and Navale S. (2015). Topical minoxidil fortified with finasteride: An account of maintenance of hair density after replacing oral finasteride. Indian Dermatology Online Journal. 6:17-20.
[https://doi.org/10.4103/2229-5178.148925]

-
Cheung LM, Cheung PC and Ooi VE. (2003). Antioxidant activity and total phenolics of edible mushroom extracts. Food Chemistry. 81: 249-255.
[https://doi.org/10.1016/S0308-8146(02)00419-3]

-
Choi YH, Cho YJ, Kim BL, Han MH, Lee HS and Jeong YG. (2021). Functional cosmetic effects of dendropanax, sea salt, and other extracts to alleviate hair loss symptoms. Asian Journal of Beauty and Cosmetology. 19:1-11.
[https://doi.org/10.20402/ajbc.2020.0100]

-
Chupa JM, Foster AM, Sumner SR, Madihally SV and Matthew HW. (2000). Vascular cell responses to polysaccharide materials: In vitro and in vivo evaluations. Biomaterials. 21:2315-2322.
[https://doi.org/10.1016/S0142-9612(00)00158-7]

-
Dal'Belo SE, Rigo Gaspar LR and Maia Campos PMBG. (2006). Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin Research and Technology. 12:241-246.
[https://doi.org/10.1111/j.0909-752X.2006.00155.x]

-
Farooq S and Sehgal A. (2018). Antioxidant activity of different forms of green tea: Loose leaf, bagged and matcha. Current Research in Nutrition and Food Science Journal. 6:35-40.
[https://doi.org/10.12944/CRNFSJ.6.1.04]

-
Greenstock CL. (1993). Radiation and aging: Free radical damage, biological response and possible antioxidant intervention. Medical Hypotheses. 41:473-482.
[https://doi.org/10.1016/0306-9877(93)90131-9]

-
Gupta AK, Talukder M and Williams G. (2022). Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia. Journal of Dermatological Treatment. 33:2946-2962.
[https://doi.org/10.1080/09546634.2022.2109567]

-
Hu Y, Xu J and Hu Q. (2003). Evaluation of antioxidant potential of Aloe vera(Aloe barbadensis Miller) extracts. Journal of Agricultural and Food Chemistry. 51:7788-7791.
[https://doi.org/10.1021/jf034255i]

-
Hwang E, Park SY, Yin CS, Kim HT, Kim YM and Yi TH. (2017). Antiaging effects of the mixture of Panax ginseng and crataegus pinnatifida in human dermal fibroblasts and healthy human skin. Journal of Ginseng Research. 41:69-77.
[https://doi.org/10.1016/j.jgr.2016.01.001]

- Im SA, Park CS and Lee CK. (2016). Immunoaugmenting activity of acemannan, the polysaccharides isolated from aloe vera gel. Korean Journal of Pharmacognosy. 47:103-109.
- Jung MH. (2019). Evaluation of antibacterial, antioxidant fractionalities and hair loss prevention effect of Platycodon grandiflorum. The Korean Journal of Food and Nutrition. 32:553-559.
-
Kim JY, Shin JY, Choi YH, Jang M, Nam YJ, Lee SY, Jeon JH, Jin MH and Lee SH. (2019). Hair growth promoting effect of Hottuynia cordata extract in cultured human hair follicle dermal papilla cells. Biological and Pharmaceutical Bulletin. 42:1665-1673.
[https://doi.org/10.1248/bpb.b19-00254]

-
Kim K. (2015). Effect of ginseng and ginsenosides on melanogenesis and their mechanism of action. Journal of Ginseng Research. 39:1-6.
[https://doi.org/10.1016/j.jgr.2014.10.006]

-
Kim MJ and Lee YA. (2018). The effect of body images about psychological stability on 20 years: Moderating effects of the between gender. Journal of Korean Society of Beauty and Art. 19:285-293.
[https://doi.org/10.18693/jksba.2018.19.2.285]

-
Kim SN, Kim SH, Hong YD, Park H, Shin SH, Kim AR and Park WS. (2015). The ginsenosides of panax ginseng promote hair growth via similar mechanism of minoxidil. Journal of Dermatological Science. 77:132-134.
[https://doi.org/10.1016/j.jdermsci.2014.12.007]

-
Kitzberger CSG, Smânia Jr, A, Pedrosa RC and Ferreira SRS. (2007). Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. Journal of Food Engineering. 80:631-638.
[https://doi.org/10.1016/j.jfoodeng.2006.06.013]

- Lee JL and Im EJ. (2009). Analysis and forecast of the domestic market for hair loss. Korean Journal of Aesthetics and Cosmetology. 7:155-163.
-
Lim JY, Kim OK, Lee JM, Lee MJ, Kang NG and Hwang JK. (2014). Protective effect of the standardized green tea seed extract on UVB-induced skin photoaging in hairless mice. Nutrition Research and Practice. 8:398-403.
[https://doi.org/10.4162/nrp.2014.8.4.398]

-
Moreno-Arrones OM, Becerra A and Vano-Galvan S. (2017). Therapeutic experience with oral finasteride for androgenetic alopecia in female-to-male transgender patients. Clinical and Experimental Dermatology. 42:743-748.
[https://doi.org/10.1111/ced.13184]

-
Park BS, Sim JB, Park SO and Noh GY. (2016). Effect of natural plant extracts on hair loss prevent in people with alopecia. Asian Journal of Dermatology. 8:8-13.
[https://doi.org/10.3923/ajd.2016.8.13]

-
Park HR, Kim GJ, Kim S and Kim KY. (2011). A screening of effective natural cosmetical materials for scalp care. Journal of Investigative Cosmetology. 7:115-122.
[https://doi.org/10.15810/jic.2011.7.2.004]

-
Pryor WA. (1986). Oxy-radicals and related species: Their formation, lifetimes, and reactions. Annual Review of Physiology. 48:657-667.
[https://doi.org/10.1146/annurev.ph.48.030186.003301]

-
Song HY, Sung NY, Jung PM, Kang MS, Park WJ and Byun EH. (2015). Whitening effect of green tea seed shell ethanol extracts. Journal of the Korean Society of Food Science and Nutrition. 44:1470-1475.
[https://doi.org/10.3746/jkfn.2015.44.10.1470]

-
Vijayalakshmi D, Dhandapani R, Jayaveni S, Jithendra PS, Rose C and Mandal AB. (2012). In vitro anti inflammatory activity of Aloe vera by down regulation of MMP-9 in peripheral blood mononuclear cells. Journal of Ethnopharmacology. 141:542-546.
[https://doi.org/10.1016/j.jep.2012.02.040]

-
Wang C, Sun J, Luo Y, Xue W, Diao H, Dong L, Chen J and Zhang J. (2006). A polysaccharide isolated from the medicinal herb Bletilla striata induces endothelial cells proliferation and vascular endothelial growth factor expression in vitro. Biotechnology Letters. 28:539-543.
[https://doi.org/10.1007/s10529-006-0011-x]

- Younus H. (2018). Therapeutic potentials of superoxide dismutase. International Journal of Health Sciences. 12:88-93.