
Probable Biosynthetic Pathways of Silymarin Precursors
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Silymarin is composed of a mixture of flavonolignans derived from secondary plant metabolism. These constituents are present in substantial amounts in milk thistle [Silybum marianum (L.) Gaertn. (Asteraceae)] seeds. Silymarin has antioxidant properties that impact it with protective effects. Because of its chemoprotective effect against liver disease, silymarin is considered a complementary and alternative hepatoprotective medicine.
Coniferyl alcohol and taxifolin, the two precursors for silymarin biosyntesis, are derived from phenylpropane and flavonoid units, respectively. Coniferyl alcohol is synthesized via the monolignol biosynthetic pathway, whereas taxifolin is synthesized via the flavonoid pathway. Multiple variables, including related substrates, production, and activating enzymes require consideration to study the biosynthetic pathway of silymarin.
This review is helpful as it summarizes the probable biosynthetic pathways of silymarin and multiple related activating enzymes and substrates found in various plants. A further understanding of silymarin is expected to increase its industrial use value.
Keywords:
Silybum marianum (L.) Gaertn, Coniferyl Alcohol, Silymarin, TaxifolinINTRODUCTION
Unlike primary metabolites, secondary metabolites are not necessary for the life cycle of plants but are synthesized in higher plants (Wink, 1988; Rhodes, 1994). Secondary metabolites are produced by interactions with the surrounding environment and other organisms, which provides efficient defense strategies against abiotic and biotic stress (Waterman, 1992).
Phytoalexins, which produce antimicrobial secondary metabolites, accumulate upon microbial exposure in plants (Rakwal et al., 1996). Isoflavonoids recognize phytoalexins and categorized flavonoids, for example, and exert a feeding deterrent activity against the larvae of scarabs and are also resistant to soil-borne fungal pathogens (Sutherland et al., 1980; Lozovaya et al., 2004).
Given the defense strategy for abiotic stress, biosynthetic flavonoids have been associated with tolerance to abiotic stress (salt, drought, and chilling stress) in other studies (Mahajan et al., 2014; Meng et al., 2015; Song et al., 2016; Wang et al., 2016). In secondary metabolites with antioxidant activity, flavonoids extracted from food, such as red grape and black tea, demonstrate an antioxidant capacity and are effective in scavenging free radicals and retarding lipid oxidants (Leung et al., 2001; María et al., 2004; Köksal et al., 2009; Hidalgo et al. 2010).
Silymarin is a mixture of constituents, including silybin, silydianin, and silychristin, a group of flavonolignans derived from secondary plants metabolites, and is well known for its antioxidant and therapeutic effects against liver disease (Flora et al., 1998; Köksal et al., 2009; Nancy et al., 2014).
Silymarin is highly accumulated in the seeds of the milk thistle [(Silybum marianum L.) Gaertn. (Asteraceae)] plant, which is native to the Mediterranean area and contains Cirsium plants, which belong to the same family as Compositae (Ma et al., 2016; Nam et al., 2018; Rodriguez et al. 2018; Kim et al. 2020; Aziz et al., 2021). In addition, regulatory gene expression analysis related to the biosynthesis of silymarin in milk thistle and Cirsium japonicum has been conducted (Roy et al., 2018; Drouet et al., 2020).
Various biosynthetic pathways and precursors exist for silymarin biosynthesis, and multiple enzymes are activated to catalyze these pathways. The probable biosynthetic pathways, and the list of phased precursors and enzymes in this pathways can be required to study related to biosynthesis in plants that retain silymarin constituents, such as milk thistle.
There are two major biosynthetic pathways for silymarin, amalgamating coniferyl alcohol and taxifolin, the precursors for silymarin biosynthesis (Yang et al., 2020). Coniferyl alcohol is synthesized via monolignol-specific pathways derived from the phenylpropanoid pathway (Vanholme et al., 2010). In the phenylpropanoid pathway, one molecule of p-coumaroyl CoA can be a precursor for the flavonoid pathway by condensing with three molecules of malonyl CoA (Dao et al., 2011). Thus, the biosynthesis of taxifolin is grouped in the flavonoid pathway from phenylpropanoids.
MATERIALS AND METHOD
In this review, the probable silymarin biosynthetic pathways and the related multiple activating enzymes and substrates that have been identified in various plants are presented to understand the biosynthesis mechanism.
RESULTS AND DISCUSSION
1. Silymarin contents
Silymarin is a pharmacologically active structural complex extracted from the seeds of milk thistle and is present in a range of 1.5% to 3% of the seed weight (Flora et a., 1998; Martin et al., 2006; Abenavoli et al., 2010).
Structural complexes are composed of flavonolignan isomers, and the major isomers include silybin (silibinin), isosilybin (isosilibinin), silychristin, isosilychristin, and silydianin (Deep et al., 2008; Valková et al., 2021). Of the isomers, the principal active compound is silybin, representing approximately 50% - 60% (Saller et al., 2001). Silybin, isosilybin, and silychristin form two diastereoisomers, namely A and B (Smith et al., 2005). Silymarin is a flavonolignans amalgamated with phenylpropane and flavonoid units (Althagafy et al., 2013; Bijak, 2017).
Phenylpropane and flavonoid units are structurally related to coniferyl alcohol and taxifolin, respectively. The structures of silymarin components are shown in Fig. 1.
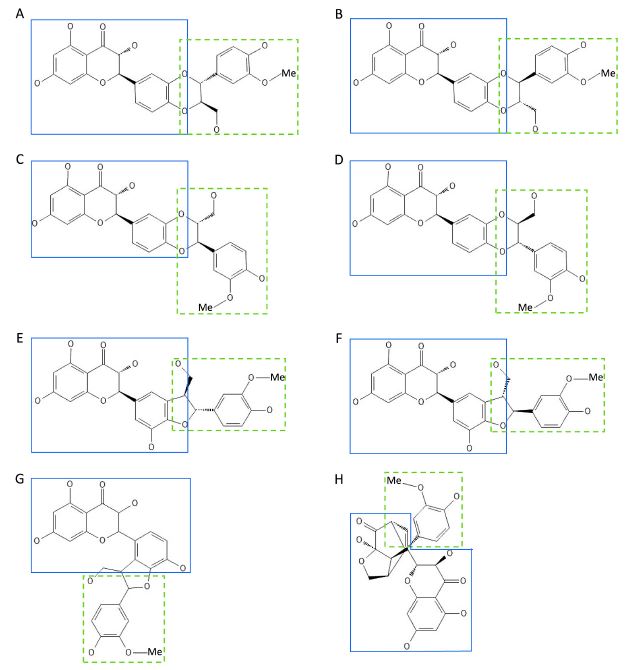
Chemical structure of various silymarin components.(A) silybin A, (B) silybin B, (C) isosilybin A, (D) isosilybin B, (E) silychristin A, (F) silychristin B, (G) isosilychristin, and (H) silydianin. Representative examples of silymarin containing flavonid (retangle by straight lines) and phenylpropane moiety (retangle by dotted lines).
2. Biosytnetic pathway of coniferyl alchool
One of the silymarin precursors, coniferyl alcohol, is synthesized via the monolignol biosynthetic pathway, which plays a major role in producing source materials for lignin biosynthesis (Wang et al., 2019).
The overall biosynthetic pathway of coniferyl alcohol is presented in Fig. 2. Monolignol belongs to one of the phenylpropanoid classes, and its pathway starts with the phenylpropanoid biochemical pathway (Vogt, 2010).
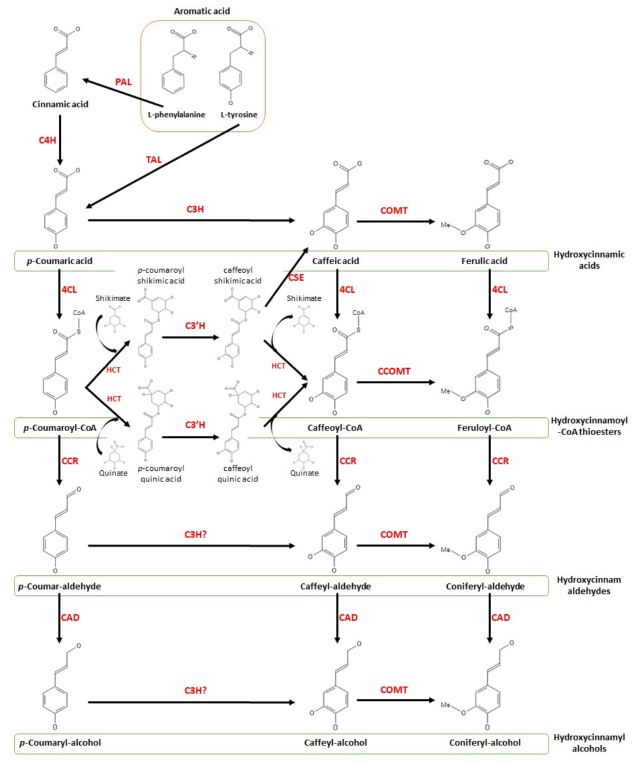
This schematic view present coniferyl alcohol biosynthetic pathway.The aromatic acids (L-phenylalanine and L-tyrosine) are converted to hydroxycinnamic acids (p-coumaric acid, caffeic acid, and ferulic acid), hydroxycinnamoyl-CoA thioesters (p-coumaroyl, caffeoyl, and feruloyl-CoA), hydroxycinnamaldehydes (p-coumaryl, caffeyl, and coniferyl-aldehyde), and hydroxycinnamyl alcohols (p-coumaryl, caffeyl, and coniferyl alcohol) by several enzymes. The enzymes involved in this pathway are phenylalanine ammonia-lyase (PAL), tyrosine ammonia-lyase (TAL), cinnamate 4-hydroxylase (C4H), p-coumarate 3-hydroxylase (C3H), p-coumaroylester 3′-hydroxylase (C3′H), caffeic acid 3-O-methyltransferase (COMT), caffeoyl-CoA 3-O-methyltransferase (CCOMT), 4-coumaric acid:coenzyme A ligase (4CL), hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase (HCT), caffeoyl shikimate esterase (CSE), cinnamoyl-CoA reductase (CCR), and cinnamyl alcohol dehydrogenase (CAD).
The phenylpropanoid pathway begins with the use of the aromatic amino acids phenylalanine and tyrosine, which are the end products of the shikimic acid pathway (Tzin and Galili, 2010; Santos-Sánchez et al., 2019).
L-Phenylalanine is catalyzed into trans-cinnamic acid by the deamination of phenylalanine ammonia-lyase (Koukol and Conn, 1961). Cinnamate 4-hydroxylase, which belongs to the CYP73A family of cytochrome P450 monooxygenases, catalyzes the p-hydroxylation of trans-cinnamic acid to yield p-coumaric acid (Hahlbrock and Scheel, 1989; Duan et al., 2004; Zhang et al., 2020). In several studies, tyrosine ammonia-lyase has also been shown to convert L-tyrosine to p-coumaric acid by deamination (Beaudoin-Eagan and Thorpe, 1985; Rosler et al., 1997; Nishiyama et al., 2010). This part, from aromatic amino acids to p-coumaric acid, is the general phenylpropanoid pathway.
The activity of p-coumarate 3-hydroxylase (C3H), which is a cytochrome P450 monooxygenase belonging to the CYP98 family, and caffeic acid 3-O-methyltransferase can transform p-coumaric acid into caffeic acid and ferulic acid, respectively (Inoue et al., 1998; Franke et al., 2002). 4-Coumaric acid: Coenzyme A ligase can convert hydroxycinnamic acids (p-coumaric acid, caffeic acid, and ferulic acid) to hydroxycinnamoyl-CoA thioesters (p-coumaroyl, caffeoyl, and feruloyl-CoA) by ligation of coenzyme A (CoA) (Chen et al., 2013).
p-Coumaroyl-CoA is treated as a precursor for the production of secondary plant metabolites, including flavonoids, and is catalyzed to caffeoyl-CoA by the two enzymes p-coumaroylester 3′-hydroxylases, which are cytochrome P450s belonging to the CYP98A3 family, and hydroxycinnamoyl-CoA: shikimate/quinate hydroxycinnamoyl transferase with shikimate/quinate, leading to p-coumaroylquinic/p-coumaroylshikimic acid and caffeoylquinic/caffeoylshikimic acid (Hoffmann et al., 2004; Boudet, 2007; Mahesh et al., 2007).
Caffeoyl CoA 3-O-methyltransferase activates caffeoyl-CoA to produce feruloyl-CoA (Inoue et al., 1998). The production of hydroxycinnamaldehydes (p-coumaryl, caffeyl, and coniferyl-aldehyde) from hydroxycinnamoyl-CoA thioesters is achieved via activation of cinnamoyl-CoA reductase (Lauvergeat et al., 2001; Vanholme et al., 2019). Cinnamyl alcohol dehydrogenase converts hydroxycinnamaldehydes to hydroxycinnamyl alcohols (p-coumaryl, caffeyl, and coniferyl alcohol) (Liu et al., 2018). Caffeic acid 3-O-methyltransferase has high activity for caffeyl-aldehyde and caffeyl-alcohol to catalyze the conversion of coniferyl-aldehyde and coniferyl-alcohol, respectively (Parvathi et al., 2001).
3. Biosysteic pathway of taxifolin
In the biosynthesis of silymarin, the phenylpropane unit (coniferyl alcohol) and flavonoid unit (taxifolin) synthesis require p-coumaryl CoA, which activates both synthesis pathways (Torres and Corchete, 2016). The taxifolin biosynthetic pathway is shown in Fig. 3.
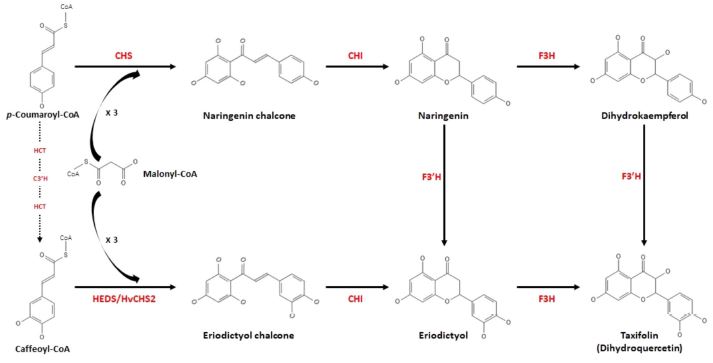
This schematic view present taxifolin biosynthetic pathway.The p-coumaroyl-CoA and caffeoyl-CoA are converted to taxifolin by several enzymes. The enzymes involved in this pathway are chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3′-hydroxylase (F3′H), and homoeriodictyol/eriodictyol synthase (HEDS/HvCHS2).
Chalcone synthase, which is a key enzyme in the flavonoid biosynthesis pathway and a member of the plant polyketide synthase family, uses one p-coumaroyl CoA as a substrate and forms naringenin chalcone by a condensation reaction with three malonyl-CoA (Flores-Sanchez, 2008; Dao et al., 2011).
Chalcone isomerase, also known as chalcone-flavanone isomerase and belonging to the class of intramolecular lyases, catalyzes the conversion of naringenin chalcone into its corresponding flavanone, naringenin (Sun et al., 2019).
Flavonoid 3′-hydroxylase (F3′H) and flavanone 3-hydroxylase (F3H) are oxoglutarate-dependent dioxygenases and cytochrome P450 hydroxylases, respectively (Dixon and Steele, 1999; Winkel-Shirley, 2001). The sequential reaction of F3′H and F3H allows the catalysis of naringenin to eriodictyol and taxifolin (dihydroquercetin) (Hammerbacher et al., 2019). In addition, the other sequential reaction of F3H and F3′H catalyze the conversion of naringenin into dihydrokaempferol and taxifolin (Brugliera et al., 1999).
In other routes to taxifolin biosynthesis, homoeriodictyol/eriodictyol synthase uses one caffeoyl-CoA as a substrate to form eriodictyol chalcone as the reaction of the chalcone synthase catalyst, and the activity of chalcone isomerase and F3H catalyzes eriodictyol chalcone into taxifolin (Christensen et al., 1998; Flores-Sanchez and Verpoorte, 2008; Morita et al., 2010; Meinert et al., 2021).
4. Amalgamation of coniferyl alcohol and taxifolin
Coniferyl alcohol and taxifolin are amalgamated via oxidative coupling for silymarin biosynthesis (flavonolignan). The oxidative coupling reaction is mediated by the formation of free radicals and is catalyzed by a peroxidase enzyme called a radical generator (AbouZid and Ahmed, 2013).
In addition, laccases (benzenediol: oxygen oxidoreductases) mediate oxidative coupling for dimerization and production of silybin (Setti et al., 1999; Gažák et al., 2008; Gavezzotti et al., 2014). A study by Lv et al. (2017) reported that silybin components of silymarin were catalyzed by ascorbate peroxidase 1 (APX1), one of the candidates for peroxidase, and APX1 showed a distinct peroxidase activity and the capacity to synthesize silybin.
Schrall and Becker (1977) reported that horseradish-peroxidase and a cell-free extract of milk thistle suspension cultures could synthesize silybin starting from coniferyl alcohol and taxifolin. In some studies, peroxidases of APX1 and horseradish-peroxidase have been used to demonstrate a green process for silybin and isosilybin production (Yang et al., 2020).
5. Others
The contents and components of silymarin biosynthesis are influenced by some factors. The study of Martin et al. (2006), use various milk thistle cultivars to present the factors influencing on contents and components of silymarin. This study show that each plant parts have different total contents and components of silymarin. The part of root have only silychristin B and silybin B components, and the two components are major in flowers. But, the contents have very low levels. In the seeds and seed heads, silychristin A, silydianin, and silybin B are the dominant components, and it have the highest total silymarin content. In addition to the difference of contents and components in each plant parts, the study show difference of contents and components depending on growth stage between the cultivars.
In the study of Liava et al. (2022), it present effects of fertilization regimes on growth, fruit, and silymarin yield in two cultivars of milk thistle. Sheep manure and calcium ammonium nitrate are used to exhibit the difference of plants growth depending on fertilization regimes. The use of manure and calcium ammonium nitrate fertilizer increase plant rosette diameter, biomass, fruit yield, and silymarin content. In this study, it is noticed that the plants growth with silymarin content can be increased based on treatment of fertilization regimes, and that the difference of silymarin content as well as silymarin composition between the two cultivars.
According to this two studies, we need to consider suitable milk thistle cultivars selection to produce silymarin as commercial viewpoint and crop management guidelines. Futhermore, the studies of Lv et al. (2017) and Torres et al. (2016) reported genes expression for silymarin biosynthetic pathway. It is regarded that molecular study for genetics is requiered using the understanding for segmented biosynthetic pathway and milk thistle cultivars which have difference of the contents and components of silymarin.
Metabolic engineering of biosynthetic pathways to produce high-value secondary metabolites as pharmaceuticals and food additives, have industrial importance using plant cell cultures, shoot cultures, root cultures, and transgenic hairy root cultures acquired through biotechnological means (Rao et al., 2002). These plant tissue cultures can be potential alternative sources for the secondary metabolites production.
The studies of Alikaridis et al. (2000) and Rahnama et al. (2008) show that production of silymarin and the components produced by the hairy root and root cultures of milk thistle. In the studies of Elwekeel et al. (2012a) and El Sherif et al. (2013), these show that silymarin accumulated through the root and shoot cultures of milk thistle can be improved by various elicitors. In addition to enhancement of silymarin production through addition of elicitors, the Elwekeel et al. (2012b) study present that the cultured cells of milk thistle have comparable cytotoxic, antioxidant, and hepato-protective effects to that of the fruits.
The study of plant tissue cultures for producing silymarin may be necessary to pharmaceutical industries. Thus, the overall understanding about silymarin biosynthetic pathways will be helpful to define efficient ways of silymarin production to utilize the various elicitors and tissue cultures.
The overall biosynthetic pathway for the silymarin components is shown in Fig. 4. The processing of the precursors (coniferyl alcohol and taxifolin) for biosynthetic pathways cannot be simply explained by one route, and the activating enzymes involved vary. In particular, the biosynthetic pathway of the precursor coniferyl alcohol, which is included in monolignol-specific pathways, has multiple routes than biosynthetic pathway of taxifolin.
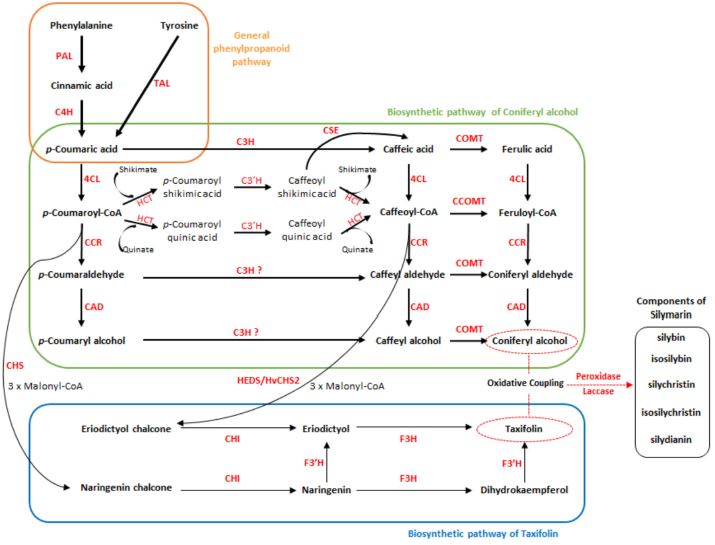
This schematic view present overall biosynthetic pathway of silymarin.In general phenylpropanoid pathway, the phenylalanine and tyrosine are converted to p-coumaric acid, and the involved enzymes are phenylalanine ammonia-lyase (PAL), tyrosine ammonia-lyase (TAL), cinnamate 4-hydroxylase (C4H). In biosynthetic pathway of coniferyl alcohol, the p-coumaric acid is converted to coniferyl alcohol, and the involved enzymes are p-coumarate 3-hydroxylase (C3H), p-coumaroylester 3′-hydroxylase (C3′H), caffeic acid 3-O-methyltransferase (COMT), caffeoyl-CoA 3-O-methyltransferase (CCOMT), 4-coumaric acid:coenzyme A ligase (4CL), hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase (HCT), caffeoyl shikimate esterase (CSE), cinnamoyl-CoA reductase (CCR), and cinnamyl alcohol dehydrogenase (CAD). The p-coumaroyl-CoA and caffeoyl-CoA are converted to naringenin chalcone and eriodictyol chalcone by chalcone synthase (CHS) and homoeriodictyol/eriodictyol synthase (HEDS/HvCHS2), respectively. In biosynthetic pathway of taxifolin, the eriodictyol chalcone and naringenin chalcone are converted to taxifolin, and the involved enzymes are chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3′-hydroxylase (F3′H). Coniferyl alcohol and taxifolin are amalgamated via oxidative coupling to formation for component of silymarin (silybin, isosilybin, silychristin, isosilychristin, and silydianin) by peroxidase and laccase.
In a study by Ha et al. (2016), caffeoyl shikimate esterase activity converted caffeoyl shikimic acid to caffeic acid in Medicago truncatula. Furthermore, the study by Liu et al. (2018) reported that cinnamyl alcohol dehydrogenases, cloned and characterized from Asarum sieboldii Miq., displayed efficient catalytic activity and substrate preference with the capability of converting aldehydes (p-coumaryl, coniferyl, and sinapyl aldehydes) to their corresponding alcohols. Regarding substrate preference, Franke et al. (2002) also reported that C3H, which is encoded by the REF8 gene isolated from Arabidopsis, had different levels of substrate activity for p-coumaric acid, p-coumaryl-aldehyde, and p-coumaryl-alcohol.
Thus, enzymes used in biosynthetic pathways in plants have substrate preferences that are activated differently on each substrate, and all plant species may not use identical enzymes for specific biosynthetic pathways. The study of biosynthetic pathways for specific secondary metabolites requires the recognition of the overall pathways with related factors (substrates, production, and activating enzymes), and an efficient activity pathway may be required in individual plants considering these factors.
Therefore, the overall biosynthetic pathway presented in this review can be utilized to study the biosynthesis of silymarin components.
Acknowledgments
This work was supported by a grant(PJ015988) from Rural Development Administration, Korea.
References
-
Abenavoli L, Capasso R, Milic N and Capasso F. (2010). Milk thistle in liver diseases: Past, present, future. Phytotherapy Research. 24:1423-1432.
[https://doi.org/10.1002/ptr.3207]

-
AbouZid S and Ahmed OM. (2013). Silymarin flavonolignans: Structure-activity relationship and biosynthesis. Studies in Natural Products Chemistry. 40:469-484.
[https://doi.org/10.1016/B978-0-444-59603-1.00014-X]

-
Alikaridis F, Papadakis D, Pantelia K and Kephalas T. (2000). Flavonolignan production from Silybum marianum transformed and untransformed root cultures. Fitoterapia. 71:379-384.
[https://doi.org/10.1016/S0367-326X(00)00134-9]

-
Althagafy HS, Meza-Aviña ME, Oberlies NH and Croatt MP. (2013). Mechanistic study of the biomimetic synthesis of flavonolignan diastereoisomers in milk thistle. The Journal of Organic Chemistry. 78:7594-7600.
[https://doi.org/10.1021/jo4011377]

-
Aziz M, Saeed F, Ahmad N, Ahmad A, Afzaal M, Hussain S, Mohamed AA, Alamri MS and Anjum FM. (2021). Biochemical profile of milk thistle(Silybum marianum L.) with special reference to silymarin content. Food Science and Nutrition. 9:244-250.
[https://doi.org/10.1002/fsn3.1990]

-
Beaudoin-Eagan LD and Thorpe TA. (1985). Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiology. 78:438-441.
[https://doi.org/10.1104/pp.78.3.438]

- Bijak M. (2017). Flavonolignans-compounds not only for liver treatment. Polski Merkuriusz Lekarski: Organ Polskiego Towarzystwa Lekarskiego. 42:34-37.
-
Boudet AM. (2007). Evolution and current status of research in phenolic compounds. Phytochemistry. 68:2722-2735.
[https://doi.org/10.1016/j.phytochem.2007.06.012]

-
Brugliera F, Barri-Rewell G, Holton TA and Mason JG. (1999). Isolation and characterization of a flavonoid 3’-hydroxylase cDNA clone corresponding to the Ht1 locus of Petunia hybrida. The Plant Journal. 19:441-451.
[https://doi.org/10.1046/j.1365-313X.1999.00539.x]

-
Chen HC, Song J, Williams CM, Shuford CM, Liu J, Wang JP, Li Q, Shi R, Gokce E, Ducoste J, Muddiman DC, Sederoff RR and Chiang VL. (2013). Monolignol pathway 4-coumaric acid: Coenzyme a ligases in populus. trichocarpa: Novel specificity, metabolic regulation, and simulation of coenzyme a ligation fluxes. Plant Physiology. 161:1501-1516.
[https://doi.org/10.1104/pp.112.210971]

-
Christensen AB, Gregersen PL, Schröder J and Collinge DB. (1998). A chalcone synthase with an unusual substrate preference is expressed in barley leaves in response to UV light and pathogen attack. Plant Molecular Biology. 37:849-857.
[https://doi.org/10.1023/A:1006031822141]

-
Dao TTH, Linthorst HJM and Verpoorte R. (2011). Chalcone synthase and its functions in plant resistance. Phytochemistry Reviews. 10:397-412.
[https://doi.org/10.1007/s11101-011-9211-7]

-
Deep G, Oberlies NH, Kroll DJ and Agarwal R. (2008). Identifying the differential effects of silymarin constituents on cell growth and cell cycle regulatory molecules in human prostate cancer cells. International Journal of Cancer. 123:41-50.
[https://doi.org/10.1002/ijc.23485]

-
Dixon RA and Steele CL. (1999). Flavonoids and isoflavonoids–a gold mine for metabolic engineering. Trends in Plant Science. 4:394-400.
[https://doi.org/10.1016/S1360-1385(99)01471-5]

-
Drouet S, Tungmunnithum D, Lainé É and Hano C. (2020). Gene expression analysis and metabolite profiling of silymarin biosynthesis during milk thistle(Silybum marianum (L.) Gaertn.) fruit ripening. International Journal of Molecular Sciences. 21:4730. https://www.mdpi.com/1422-0067/21/13/4730, (cited by 2022 June 15).
[https://doi.org/10.3390/ijms21134730]

-
Duan H, Civjan NR, Sligar SG and Schuler MA. (2004). Co-incorporation of heterologously expressed Arabidopsis cytochrome P450 and P450 reductase into soluble nanoscale lipid bilayers. Archives of Biochemistry and Biophysics. 424:141-153.
[https://doi.org/10.1016/j.abb.2004.02.010]

-
Elwekeel A, AbouZid S, Sokkar N and Elfishway A. (2012b). Studies on flavanolignans from cultured cells of Silybum marianum. Acta Physiologiae Plantarum. 34:1445-1449.
[https://doi.org/10.1007/s11738-012-0942-x]

-
Elwekeel A, Elfishway A and AbouZid S. (2012a). Enhanced accumulation of flavonolignans in Silybum marianum cultured roots by methyl jasmonate. Phytochemistry Letters. 5:393-396.
[https://doi.org/10.1016/j.phytol.2012.03.010]

-
Flora K, Hahn M, Rosen H and Benner K. (1998). Milk thistle (Silybum marianum L.) for the therapy of liver disease. The American Journal of Gastroenterology. 93:139-143.
[https://doi.org/10.1111/j.1572-0241.1998.00139.x]

-
Flores-Sanchez IJ and Verpoorte R. (2008). Secondary metabolism in cannabis. Phytochemistry Reviews. 7:615-639.
[https://doi.org/10.1007/s11101-008-9094-4]

- Flores-Sanchez IJ. (2008). Polyketide synthases in Cannabis sativa L. Leiden University. Leiden, Netherlands. 7:615-639.
-
Franke R, Humphreys JM, Hemm MR, Denault JW, Ruegger MO, Cusumano JC and Chapple C. (2002). The Arabidopsis REF8 gene encodes the 3-hydroxylase of phenylpropanoid metabolism. The Plant Journal. 30:33-45.
[https://doi.org/10.1046/j.1365-313X.2002.01266.x]

-
García-Alonso M, Rimbach G, Rivas-Gonzalo JC and de Pascual-Teresa S. (2004). Antioxidant and cellular activities of anthocyanins and their corresponding vitisins a studies in platelets, monocytes, and human endothelial cells. Journal of Agricultural and Food Chemistry. 52:3378-3384.
[https://doi.org/10.1021/jf035360v]

-
Gavezzotti P, Vavříková E, Valentová K, Fronza G, Kudanga T, Kuzma M, Riva S, Biedermann D and Křen V. (2014). Enzymatic oxidative dimerization of silymarin flavonolignans. Journal of Molecular Catalysis B: Enzymatic. 109:24-30.
[https://doi.org/10.1016/j.molcatb.2014.07.012]

-
Gažák R, Sedmera P, Marzorati M, Riva S and Křen V. (2008). Laccase-mediated dimerization of the flavonolignan silybin. Journal of Molecular Catalysis B: Enzymatic. 50:87-92.
[https://doi.org/10.1016/j.molcatb.2007.09.005]

-
Ha CM, Escamilla-Trevino L, Yarce JCS, Kim H, Ralph J, Chen F and Dixon RA. (2016). An essential role of caffeoyl shikimate esterase in monolignol biosynthesis in Medicago truncatula. The Plant Journal. 86:363-375.
[https://doi.org/10.1111/tpj.13177]

-
Hahlbrock K and Scheel D. (1989). Physiology and molecular biology of phenylpropanoid metabolism. Annual Review of Plant Physiology and Plant Molecular Biology. 40:347-369.
[https://doi.org/10.1146/annurev.pp.40.060189.002023]

-
Hammerbacher A, Kandasamy D, Ullah C, Schmidt A, Wright LP and Gershenzon J. (2019). Flavanone-3-hydroxylase plays an important role in the biosynthesis of spruce phenolic defenses against bark beetles and their fungal associates. Frontiers in Plant Science. 10:208. https://www.frontiersin.org/articles/10.3389/fpls.2019.00208/full (cited by 2022 June 15).
[https://doi.org/10.3389/fpls.2019.00208]

-
Hidalgo M, Sánchez-Moreno C and de Pascual-Teresa S. (2010). Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chemistry. 121:691-696.
[https://doi.org/10.1016/j.foodchem.2009.12.097]

-
Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, Lapierre C, Pollet B and Legrand M. (2004). Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. The Plant Cell. 16:1446-1465.
[https://doi.org/10.1105/tpc.020297]

-
Inoue K, Sewalt VJ, Murray Ballance G, Ni W, Stürzer C and Dixon RA. (1998). Developmental expression and substrate specificities of alfalfa caffeic acid 3-O-methyltransferase and caffeoyl coenzyme A 3-O-methyltransferase in relation to lignification. Plant Physiology. 117:761-770.
[https://doi.org/10.1104/pp.117.3.761]

- Kim JR, Paje LA, Choi JW, Lee HD, Shim JS, Shim JH, L. Geraldino PJ and Lee SH. (2020). Determination of silymarin and silybin diastereomers in Korean milk thistle using HPLC/UV Analysis. Korean Journal of Pharmacognosy. 51:297-301.
-
Köksal E, Gülçin I, Beyza S, Sarikaya O and Bursal E. (2009). In vitro antioxidant activity of silymarin. Journal of Enzyme Inhibition and Medicinal Chemistry. 24:395-405.
[https://doi.org/10.1080/14756360802188081]

-
Koukol J and Conn EE. (1961). The metabolism of aromatic compounds in higher plants: IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. Journal of Biological Chemistry. 236:2692-2698.
[https://doi.org/10.1016/S0021-9258(19)61721-7]

-
Lauvergeat V, Lacomme C, Lacombe E, Lasserre E, Roby D and Grima-Pettenati J. (2001). Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during development and in response to infection with pathogenic bacteria. Phytochemistry. 57:1187-1195.
[https://doi.org/10.1016/S0031-9422(01)00053-X]

-
Leung LK, Su Y, Chen R, Zhang Z, Huang Y and Chen ZY. (2001). Theaflavins in black tea and catechins in green tea are equally effective antioxidants. The Journal of Nutrition. 131:2248-2251.
[https://doi.org/10.1093/jn/131.9.2248]

-
Liava V, Karkanis A, Danalatos N and Tsiropoulos N. (2022). Effects of two varieties and fertilization regimes on growth, fruit, and silymarin yield of milk thistle crop. Agronomy. 12:105. https://www.mdpi.com/2073-4395/12/1/105, (cited by 2022 June 15).
[https://doi.org/10.3390/agronomy12010105]

-
Liu J, Xu C, Zhang H, Liu F, Ma D and Liu Z. (2018). Comparative transcriptomics analysis for gene mining and identification of a cinnamyl alcohol dehydrogenase involved in methyleugenol biosynthesis from Asarum sieboldii Miq. Molecules. 23:3184. https://www.mdpi.com/1420-3049/23/12/3184, (cited by 2022 June 15).
[https://doi.org/10.3390/molecules23123184]

-
Lozovaya VV, Lygin AV, Zernova OV, Li S, Hartman GL and Widholm JM. (2004). Isoflavonoid accumulation in soybean hairy roots upon treatment with Fusarium solani. Plant Physiology and Biochemistry. 42:671-679.
[https://doi.org/10.1016/j.plaphy.2004.06.007]

-
Lv Y, Gao S, Xu S, Du G, Zhou J and Chen J. (2017). Spatial organization of silybin biosynthesis in milk thistle[Silybum marianum(L.) Gaertn]. The Plant Journal. 92:995-1004.
[https://doi.org/10.1111/tpj.13736]

-
Ma Q, Wang LH and Jiang JG. (2016). Hepatoprotective effect of flavonoids from Cirsium japonicum DC on hepatotoxicity in comparison with silymarin. Food and Function. 7:2179-2184.
[https://doi.org/10.1039/C6FO00068A]

-
Mahajan M and Yadav SK. (2014). Overexpression of a tea flavanone 3-hydroxylase gene confers tolerance to salt stress and Alternaria solani in transgenic tobacco. Plant Molecular Biology. 85:551-573.
[https://doi.org/10.1007/s11103-014-0203-z]

-
Mahesh V, Million-Rousseau R, Ullmann P, Chabrillange N, Bustamante J, Mondolot L, Morant M, Noirot M, Hamon S, de Kochko A, Werck-Reichhart D and Campa C. (2007). Functional characterization of two p-coumaroyl ester 3′-hydroxylase genes from coffee tree: Evidence of a candidate for chlorogenic acid biosynthesis. Plant Molecular Biology. 64:145-159.
[https://doi.org/10.1007/s11103-007-9141-3]

-
Martin RJ, Lauren DR, Smith WA, Jensen DJ, Deo B and Douglas JA. (2006). Factors influencing silymarin content and composition in variegated thistle(Silybum marianum). New Zealand Journal of Crop and Horticultural Science. 34:239-245.
[https://doi.org/10.1080/01140671.2006.9514413]

-
Meinert H, Yi D, Zirpel B, Schuiten E, Geißler T, Gross E, Brückner SI, Hartmann B, Röttger C, Ley JP and Bornscheuer UT. (2021). Discovery of novel bacterial chalcone isomerases by a sequence-structure-function-evolution strategy for enzymatic synthesis of (S)-flavanones. Angewandte Chemie International Edition. 60:16874-16879.
[https://doi.org/10.1002/anie.202107182]

-
Meng C, Zhang S, Deng YS, Wang GD and Kong FY. (2015). Overexpression of a tomato flavanone 3-hydroxylase-like protein gene improves chilling tolerance in tobacco. Plant Physiology and Biochemistry. 96:388-400.
[https://doi.org/10.1016/j.plaphy.2015.08.019]

-
Morita H, Abe I and Noguchi H. (2010). Plant type III PKS. Comprehensive natural products II: Chemistry and Biology. Elsvier press. Amsterdam, Netherlands. 1:171-226.
[https://doi.org/10.1016/B978-008045382-8.00022-8]

-
Nam SH, Lee BH and Kim YJ. (2018). Silymarin contents and liver protection effects of six domestic cultivated thistles. Trends in Agriculture and Life Sciences. 56:55-62.
[https://doi.org/10.29335/tals.2018.56.55]

-
Nishiyama Y, Yun CS, Matsuda F, Sasaki T, Saito K and Tozawa Y. (2010). Expression of bacterial tyrosine ammonia-lyase creates a novel p-coumaric acid pathway in the biosynthesis of phenylpropanoids in Arabidopsis. Planta. 232:209-218.
[https://doi.org/10.1007/s00425-010-1166-1]

-
Parvathi K, Chen F, Guo D, Blount JW and Dixon RA. (2001). Substrate preferences of O-methyltransferases in alfalfa suggest new pathways for 3-O-methylation of monolignols. The Plant Journal. 25:193-202.
[https://doi.org/10.1111/j.1365-313X.2001.00956.x]

-
Rahnama H, Hasanlou T, Shams MR and Sepehrifar R. (2008). Silymarin production by hairy root culture of Silybum marianum (L.) Gaertn. Iranian Journal of Biotechnology. 6:113-118
[https://doi.org/10.1055/s-0028-1084652]

- Rakwal R, Tamogami S and Kodama O. (1996). Role of jasmonic acid as a signaling molecule in copper chloride-elicited rice phytoalexin production. Bioscience, Biotechnology, and Biochemistry. 60:1046-1048.
-
Rao SR and Ravishankar GA. (2002). Plant cell cultures: Chemical factories of secondary metabolites. Biotechnology Advances. 20:101-153.
[https://doi.org/10.1016/S0734-9750(02)00007-1]

-
Rhodes MJC. (1994). Physiological roles for secondary metabolites in plants: Some progress, many outstanding problems. Plant Molecular Biology. 24:1-20.
[https://doi.org/10.1007/BF00040570]

-
Rodriguez JP, Quilantang NG, Lee JS, Lee JM, Kim HY, Shim JS and Lee SH. (2018). Determination of silybin B in the different parts of Silybum marianum using HPLC-UV. Natural Product Sciences. 24:82-87.
[https://doi.org/10.20307/nps.2018.24.2.82]

-
Rosler J, Krekel F, Amrhein N and Schmid J. (1997). Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiology. 113:175-179.
[https://doi.org/10.1104/pp.113.1.175]

-
Roy NS, Kim JA, Choi AY, Ban YW, Park NI, Park KC, Yang HS, Choi IY and Kim SO. (2018). RNA-Seq De Novo assembly and differential transcriptome analysis of Korean medicinal herb Cirsium japonicum var. spinossimum. Genomics and Informatics. 16:e34. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6440657/, (cited by 2022 June 15).
[https://doi.org/10.5808/GI.2018.16.4.e34]

-
Saller R, Meier R and Brignoli R. (2001). The use of silymarin in the treatment of liver diseases. Drugs. 61:2035-2063.
[https://doi.org/10.2165/00003495-200161140-00003]

-
Santos-Sánchez NF, Salas-Coronado R, Hernández-Carlos B and Villanueva-Cañongo C. (2019). Shikimic acid pathway in biosynthesis of phenolic compounds. In Sato-Hernàndez M. et al. (ed.). Plant physiological aspects of phenolic compounds. Intech open. London, England. p.35-50.
[https://doi.org/10.5772/intechopen.83815]

-
Schrall R and Becker H. (1977). Callus–UND suspensionskulturen von Silybum marianum. Planta Medica. 32:27-32.
[https://doi.org/10.1055/s-0028-1097554]

-
Setti L, Giuliani S, Spinozzi G and Pifferi PG. (1999). Laccase catalyzed-oxidative coupling of 3-methyl 2-benzothiazolinone hydrazone and methoxyphenols. Enzyme and Microbial Technology. 25:285-289.
[https://doi.org/10.1016/S0141-0229(99)00059-9]

-
Sherif EF, Khattab S, Ibrahim AK and Ahmed SA. (2013). Improved silymarin content in elicited multiple shoot cultures of Silybum marianum L. Physiology and Molecular Biology of Plants. 19:127-136.
[https://doi.org/10.1007/s12298-012-0141-7]

-
Smith WA, Lauren DR, Burgess EJ, Perry NB and Martin RJ. (2005). A silychristin isomer and variation of flavonolignan levels in milk thistle(Silybum marianum) fruits. Planta Medica. 71:877-880.
[https://doi.org/10.1055/s-2005-864187]

-
Song X, Diao J, Ji J, Wang G, Guan C, Jin C and Wang, Y. (2016). Molecular cloning and identification of a flavanone 3-hydroxylase gene from Lycium chinense, and its overexpression enhances drought stress in tobacco. Plant Physiology and Biochemistry. 98:89-100.
[https://doi.org/10.1016/j.plaphy.2015.11.011]

-
Sun W, Shen H, Xu H, Tang X, Tang M, Ju Z and Yi Y. (2019). Chalcone isomerase a key enzyme for anthocyanin biosynthesis in Ophiorrhiza japonica. Frontiers in Plant Science. 10:865. https://www.frontiersin.org/articles/10.3389/fpls.2019.00865/full, (cited by 2022 June 15).
[https://doi.org/10.3389/fpls.2019.00865]

-
Sutherland ORW, Russell GB, Biggs DR and Lane GA. (1980). Insect feeding deterrent activity of phytoalexin isoflavonoids. Biochemical Systematics and Ecology. 8:73-75.
[https://doi.org/10.1016/0305-1978(80)90029-0]

-
Torres M and Corchete P. (2016). Gene expression and flavonolignan production in fruits and cell cultures of Silybum marianum. Journal of Plant Physiology. 192:111-117.
[https://doi.org/10.1016/j.jplph.2016.02.004]

-
Tzin V and Galili G. (2010). New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Molecular Plant. 3:956-972.
[https://doi.org/10.1093/mp/ssq048]

-
Valková V, Ďúranová H, Bilčíková J and Habán M. (2020). Milk thistle(Silybum marianum): A valuable medicinal plant with several therapeutic purposes. Journal of Microbiology, Biotechnology and Food Sciences. 9:836-843.
[https://doi.org/10.15414/jmbfs.2020.9.4.836-843]

-
Vanholme R, De Meester B, Ralph J and Boerjan W. (2019). Lignin biosynthesis and its integration into metabolism. Current Opinion in Biotechnology. 56:230-239.
[https://doi.org/10.1016/j.copbio.2019.02.018]

-
Vanholme R, Demedts B, Morreel K, Ralph J and Boerjan W. (2010). Lignin biosynthesis and structure. Plant Physiology. 153:895-905.
[https://doi.org/10.1104/pp.110.155119]

- Vargas-Mendoza N, Madrigal-Santillán E, Morales-González Á, Esquivel-Soto J, Esquivel-Chirino C, y González-Rubio MGL, Gayosso-de-Lucio JA and Morales-González JA. (2014). Hepatoprotective effect of silymarin. World Journal of Hepatology. 6:144-149.
-
Vogt T. (2010). Phenylpropanoid biosynthesis. Molecular Plant. 3:2-20.
[https://doi.org/10.1093/mp/ssp106]

-
Wang F, Kong W, Wong G, Fu L, Peng R, Li Z and Yao Q. (2016). AtMYB12 regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis thaliana. Molecular Genetics and Genomics. 291:1545-1559.
[https://doi.org/10.1007/s00438-016-1203-2]

-
Wang JP, Liu B, Sun Y, Chiang VL and Sederoff RR. (2019). Enzyme-enzyme interactions in monolignol biosynthesis. Frontiers in Plant Science. 9:1942. https://www.frontiersin.org/articles/10.3389/fpls.2018.01942/full, (cited by 2022 June 15).
[https://doi.org/10.3389/fpls.2018.01942]

- Waterman PG. (1992). Roles for secondary metabolites in plants. In Derek J. et al. (ed.). Ciba foundation symposium 171 - secondary metabolites: Their function and evolution: Secondary Metabolites: Their function and evolution: Ciba foundation symposium 171. John Wiley and Sons Ltd. Chichester, England. p.269-275.
-
Wink M. (1988). Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theoretical and Applied Genetics. 75:225-233.
[https://doi.org/10.1007/BF00303957]

-
Winkel-Shirley B. (2001). Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiology. 126:485-493.
[https://doi.org/10.1104/pp.126.2.485]

-
Yang J, Liang J, Shao L, Liu L, Gao K, Zhang JL, Sun Z, Xu W, Lin P, Yu R and Zi J. (2020). Green production of silybin and isosilybin by merging metabolic engineering approaches and enzymatic catalysis. Metabolic Engineering. 59:44-52.
[https://doi.org/10.1016/j.ymben.2020.01.007]

-
Zhang B, Lewis KM, Abril A, Davydov DR., Vermerris W, Sattler SE and Kang C. (2020). Structure and function of the cytochrome P450 monooxygenase cinnamate 4-hydroxylase from sorghum bicolor. Plant Physiology. 183:957-973.
[https://doi.org/10.1104/pp.20.00406]
