
Extending the Storage Periods of Zanthoxylum schinifolium Seed Oil using Sodium Bicarbonate and Ascorbic Acid
 ; Seung Mi Kang2
; Seung Mi Kang2 ; Seong Hyeon Yong3
; Seong Hyeon Yong3 ; Yu Won Seol4
; Yu Won Seol4 ; Do Hyeon Kim5 ; Jun Ho Park6
; Do Hyeon Kim5 ; Jun Ho Park6 ; Chan Yeol Yu7
; Chan Yeol Yu7 ; Myung Suk Choi8, †
; Myung Suk Choi8, †
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The seed oil of Zanthoxylum schinifolium S. et Z. (sancho) is a traditional cooking oil that has long been sold at a very high price however, depending on the method of extraction and storage, this oil becomes rancid occurs very quickly. Therefore, this study aimed to find a material that prevents rancidity and improves the storage properties of sancho oil.
Sancho oil was extracted using an extraction press, and acid values were compared with commercially available vegetable oils, sancho oil had a higher acid value than other vegetable oils. A very high acid value was observed in sancho oil stored for 6 months, regardless of temperature, requiring an effective storage method. The high acid value and the decrease in turbidity of sancho oil are dependent on the days of sedimentation. Treatment with sodium bicarbonate by concentration resulted in minimal changes in acid value over time. However, minor differences were detected among the treatment concentrations. Ascorbic acid was added to maximize the effect of sodium bicarbonate, and it was observed that ascorbic acid did not improve the antioxidant effect. The sodium bicarbonate and ascorbic acid mixture resulted in minimal change in acid value at temperature up to 25℃.
Sancho oil becomes rancid very quicky and requires efficient storage techniques. Sodium bicarbonate and ascorbic acid have been proven to be useful as safe anti-racidity agents without causing harm to humans.
Keywords:
Zanthoxylum schinifolium S. et Z., Acid Value, Antioxidant, Rancidity, Sancho OilINTRODUCTION
Sancho (Zanthoxylum schinifolium S. et Z.) is a deciduous shrub belonging to the Rutaceae family. As an aromatic plant resource, it grows widely in Korea, China, Japan, Taiwan, and Manchuria, and grows well in the foothills of the country (Lee, 1998). Z. schinifolium was one of the spices before red pepper was used, and in Gangwon-do, it was widely used for powdered spices, pickles, medicinal and vegetable oil products (Jeong and Shin, 1990).
Plant oils are limited in their use due to changes that occur during oil expression, cooking and storage. In general, vegetable oils are oxidized according to the oil expression method and storage period, and such changes can cause serious problems in food hygiene by generating harmful substances (Song et al., 2003).
Sancho has effects such as scented dry stomach, anti-inflammatory, and diuretic, and is used as an anthelmintic and preservative. Besides detoxification, it has a wide variety of uses in the a folk remedy as a treatment for appetite enhancement, toothache, neuralgia, poor circulation, cold, and stroke (Lee, 1996).
Sancho has chemical composition (Chung, 2005; Chang and Kim, 2008), antibacterial activity (Seo et al., 1999; Park et al., 2008), insecticidal effect (Choochote et al., 2007), anti-inflammatory effect (Lee et al., 2009) etc. have been studied. However, most of these studies are limited to the investigation of the biological activities of the bark, leaves and fruits of the Sancho tree, so studies on sancho oil are very insufficient.
Sancho oil is a traditional cooking oil that has long been sold at very high prices. However, it has been reported that sancho oil bursts very quickly depending on the method of extraction and storage (Kim et al., 2012; Kang et al., 2017). And now there is no proper way to prevent rancidity of sancho oil. Therefore, it is important to find an appropriate method to prevent rancidity of the sancho oil. In particular, it is very important to search for materials that are not harmful to the human body and are inexpensive. Antioxidants have been proposed to increase the storage properties of fats and oils, but synthetic antioxidants have problems with human safety, so it is necessary to find a material that can secure human safety.
Although the cultivation area of Sancho tree is small enough to not be captured by statistics, tree planting has been rapidly increasing due to the trend of well-being. Currently, Cultivar Hancho10 is registered as a new variety, and the market value is 215.3 billion won, the technology value is 40.9 billion won, and the ripple effect is analyzed to reach 670 billion won (NFSVC, 2017). However, sancho oil is produced from the folk medicinal plant, Sancho tree, and research is urgently needed to increase storage properties. This study was conducted to develop a material that can overcome the problem of rancidity using a material that is safe for the human body along with more efficient purification of sancho oil.
MATERIALS AND METHODS
1. Experimental materials
The sample used for the study was used to oil extracted from a sancho (Zanthoxylum schinifolium S. et Z.) tree farm located in Hadong from Korea. Sancho seed-pods were collected on 5th October, 2018 and then the oil was immediately extracted from them.
The extraction of Sancho oil was performed by the compression method. After picking the Sancho seeds, they were extracted with a expella pressing machine (Ubo Sancho, Milyang, Korea). The oil was cooled at room temperature and centrifuged at 2,500 rpm for 5 minutes using a centrifuge. Milked sancho oil was stored at 5℃ until use. The acid value of the sancho oil extracted directly from the compression-type expeller extraction machine was between 1.0 and 2.0.
2. Measurement of the acid value of vegetable oils
The acid values of sancho oil and other vegetable oils were compared. The acid value was investigated for commercially available edible oils such as olive oil, grape seed oil, canola oil and sesame oil.
Acid value of sancho oil and blending oil was measured according to American Oil Chemists` Society (AOCS, 1990). 1.0 g of the sample was taken in an Erlenmeyer flask and mixed with 1 : 2 (v/v) ethanol (Sigma-Aldrich Co., St. Louis, MO, USA) 20 ㎖ was added to dissolve the sancho oil. The Phenolphthalein solution (Sigma-Aldrich Co., St. Louis, MO, USA) was used as an indicator and titrated with 0.1 N potassium hydroxide (KOH) (Sigma-Aldrich Co., St. Louis, MO, USA) until the ethanol solution became pale red. The acid value was determined by the following equation, and the experiment was repeated three times. The acidity was measured in an incubator (SH-75B, Seyoung Scientific Co., Bucheon, Korea) adjusted to 25℃.
Acid value = 5.611 × A × F / sample weight (g)
A: Volume of 0.1 N KOH (㎖), F: Tilter of 0.1 N KOH (㎖)
3. Measurement of change in acid value after 6 months storage
50 ㎖ sancho oil was put in a 100 ㎖ plastic container, put at -17℃, 4℃, and 25℃, respectively and stored in the dark for 6 months. The stored sancho oil was taken out of each incubator and the acid value was measured by the method mentioned above.
4. Detemination of acid value and turbidity according to sedimentation
50 ㎖ of sancho oil was taken and placed in a erlenmeyer flask and left at 25℃. for 7 days, and the turbidity and acid value were measured.
To measure turbidity, take 0.5 ㎖ of a oil in a 1000 ㎖ erlenmeyer flask, dilute it in a ratio of 1 : 1000 with 0.1% sodium dodecyl sulfate (SDS) solution, and stir it with a homogenizer (Ultra-Turrax T25, IKA Laboretechnik Co., Staufen, Germany) for 3 minutes. After leaving for 2 hours, absorbance was measured at 500 ㎚ with a spectrophotometer (PR-101a, Atago Co., Ltd., Tokyo, Japan) and expressed as turbidity.
The acid value measurement according to sedimentation was performed by the above method.
5. Determination of the effect of sodium bicarbonate treatment
20 ㎖ of sancho oil was placed in a 50 ㎖ conical tube, and sodium bicarbonate (NaHCO3) was added at concentrations of 0.5, 1.0, and 2%. The oil was placed in a dark place at 25 ± 2 ℃, and the acid value was measured every 5 days.
6. Statistical analysis
All experiments were performed 3 times. Data are presented as means and standard deviation and analyzed by one-way ANOVA using the IBM SPSS statistical package (Ver. 24, IBM Co., Armonk, NY, USA). Means were compared at 5% significance level using Duncan’'s Multiple Range Test (DMRT) comparison (p < 0.05).
RESULTS AND DISCUSSION
1. Acid value of vegetable oils
The acid values of four commercially available cooking oils and sancho (Zanthoxylum schinifolium S. et Z.) oil were investigated (Fig. 1). The acid values of olive oil and grape seed oil were 0.44 and 0.42, respectively, 0.06 for canola oil and 1.81 for sesame oil, and 2.53 for sancho oil. Unlike other edible oil, sesame oil and sancho oil sold without degumming exhibited relatively high acid values.
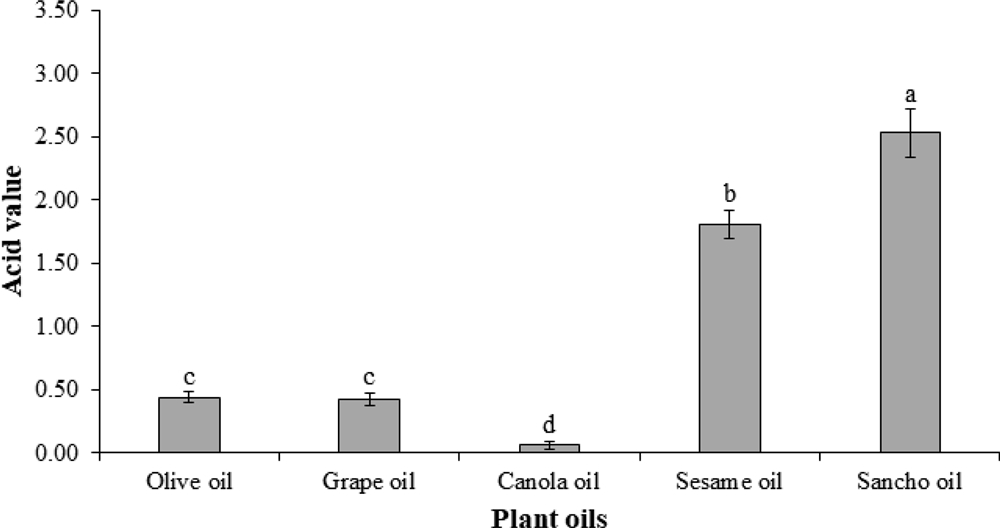
Acid values of sancho oil and commercial vegetable oils.1.0 g of sancho oil and vegetable oil were taken and placed in an Erlenmeyer flask and the acid value was measured by the method of AOCS (1990). Data represent the means ± S.E of three replicates. *Different letter for each treatment shows significant difference using Duncan’s Multiple Range Test (DMRT) comparison (p < 0.05).
2. Acid value after 6 months of storage of sancho oil
Sancho oil stored for 6 months had different acid values depending on the temperature (Fig. 2).
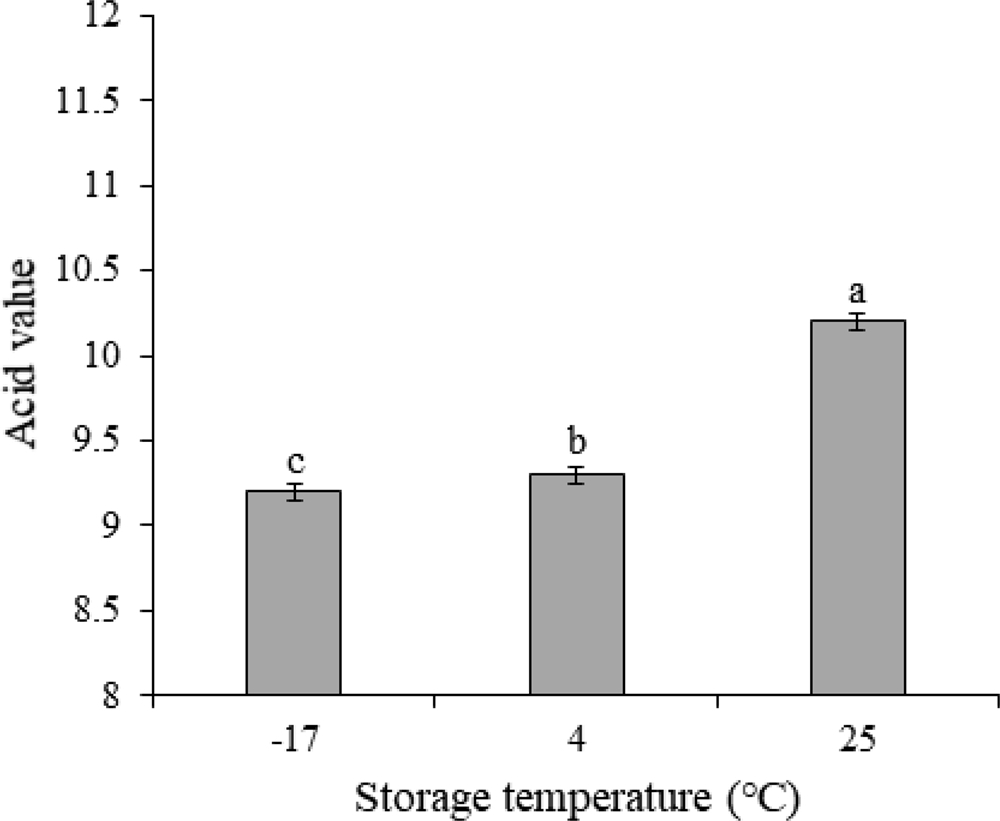
Changes in acid value of sancho oil by storage temperature.50 ㎖ of sancho oil extracted by expella method were taken, placed in an erlenmeyer flask, placed at -17℃, 4℃, and 25℃ for 6 months, and the acid value was measured by the method of AOCS (1990). Data represent the means ± S.E of three replicates. *Different letter for each treatment shows significant difference using Duncan’s Multiple Range Test (DMRT) comparison (p < 0.05).
The acid value of sancho oil was lowest at -17℃ among the three storage temperatures, and the acid value increased as the temperature increased. Sancho oil stored at room temperature has a very high acid value. According to the Food Code (KFDA 2007), when fresh soybean oil and canola oil are generally released, the acid value is 0.6 or less, shortening is 0.8 or less, extra virgin (oil squeezed from fruit) and pure olive oil is 2 or less, and the standard for fried fat is 3. Therefore, it seemed that sancho oil could not be used if stored without treatment such as antioxidants and degumming.
3. Acid value and turbidity according to sedimentation of sancho oil
The acid value of sancho oil tended to increase as the number of days left to stand at room temperature to settle the sediment increased (Fig. 3). The acid value of sancho oil gradually increased until the 4th day and then increased rapidly after that. On the other hand, turbidity decreased sharply after starting sedimentation, and decreased relatively slowly thereafter.
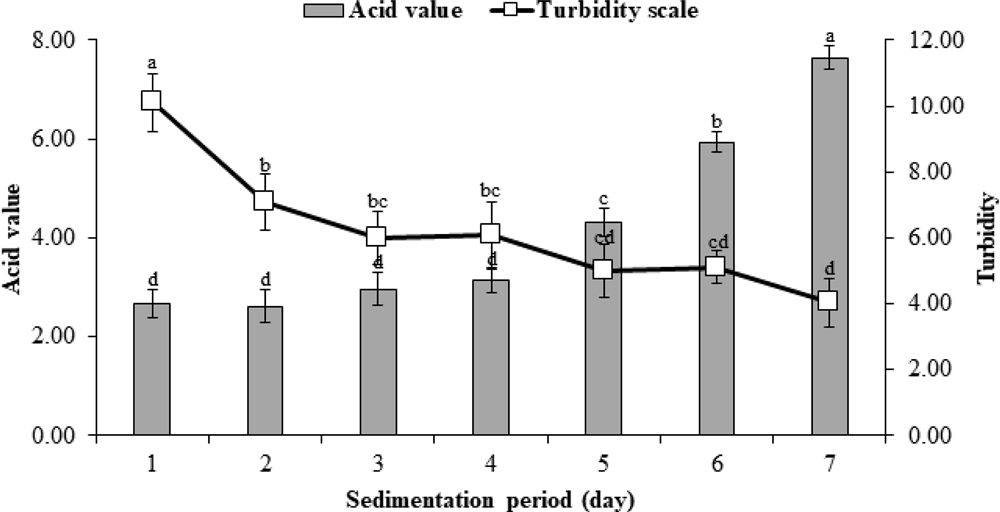
Changes in acid value and turbidity according to the sedimentation period of sancho oil.50 ㎖ of sancho oil was taken and placed in a Erlenmeyer flask and left at 25℃. for 7 days, and the turbidity and acid value were measured. *Different letter for each treatment shows significant difference using Duncan’s Multiple Range Test (DMRT) comparison (p < 0.05).
As for vegetable oils including sancho oil, the acid value changes as the sedimentation days pass. In this study, the change in acid value of sancho oil occurred much faster than that of perilla and soybean oil. The acid values of perilla and soybean oil increased after 4 days of standing and increased relatively rapidly after 8 days of culture (Lee et al., 2014). It was reported that the difference in rancidity of the vegetable oil depends on the fatty acid composition. In particular, the content of linolenic acid among unsaturated fatty acids is known to play a role of very lowering oxidation stability.
The major constituent fatty acids of sancho oil were eicosenoic acid 30.88%, linoleic acid 23.4%, oleic acid 19.94%, palmitic acid 10.52%, and n-9 monounsaturated fatty acids accounted for more than 60% (Cha et al., 2000). Kim et al. (2012) reported that oleic acid accounted for about 30% of the total fatty acids, followed by linoleic acid and linolenic acid, accounting for 27% and 20%, respectively.
In this study, it was found that rancidity and turbidity increased as the sedimentation period passed. This implies that effective anti-runtime methods and purification techniques are needed.
These impurities can act as a factor of rancidity during the storage of pepper oil. In general, purification of oils and fats goes through a degumming and neutralization process (Kang et al., 2017). As a prior study, our research team has de-gathered and refined sancho oil. The degumming process is a method of removing phospholipids (lecithin) dissolved in fats and oils, and the deoxidation process is a process of removing coexisting phospholipids (lecithin) by neutralizing the free fatty acids contained in crude oil with an aqueous alkali solution (NaOH).
4. Effect of preventing rancidity by treatment with sodium bicarbonate
The acid value of the sancho oil started to increase from the 5th day after being placed at 25℃, and the acid value started to increase rapidly from the 20th day. However, no increase in acid value was observed with sodium bicarbonate treatment. There was no increase in acid value regardless of the sodium bicarbonate treatment concentration (Fig. 4). Therefore, it can be seen that sodium bicarbonate is a material that delays rancidity of sancho oil.
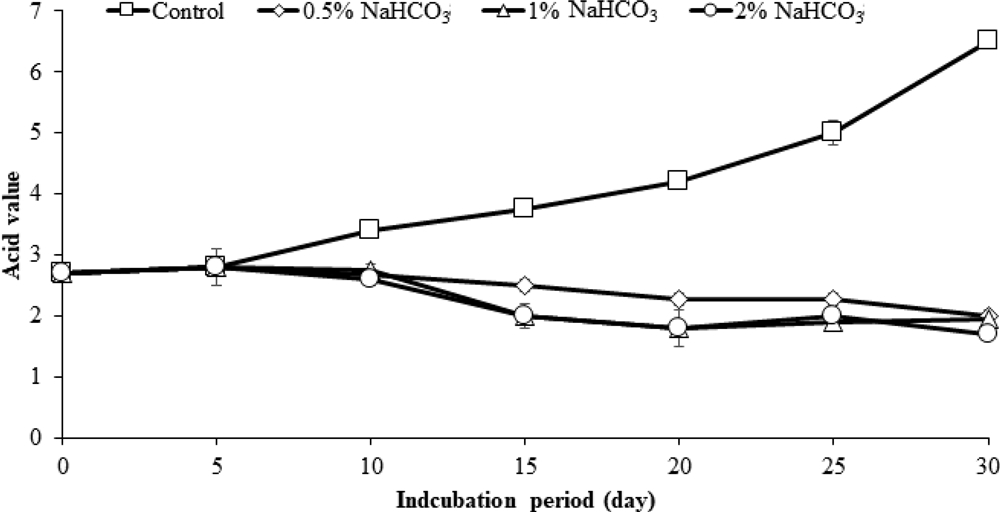
Changes in acid value depending on the concentration of sodium bicarbonate treatment.Sancho oil to which sodium bicarbonate was added was placed in an incubator at 25℃ and the acid value and turbidity were measured.
Changes in acid value and turbidity were observed by mixing sodium bicarbonate and ascorbic acid (Table 1, Fig. 5.). The acid value of the ascorbic acid treatment decreased as the concentration increased compared to the untreated group. Also, when sodium bicarbonate and ascorbic acid were mixed, the acid value was low. However, the mixed treatment of sodium bicarbonate and ascorbic acid did not show a significant difference according to the concentration. It was recognized that mixing sodium bicarbonate and ascorbic acid could prevent rancidity.
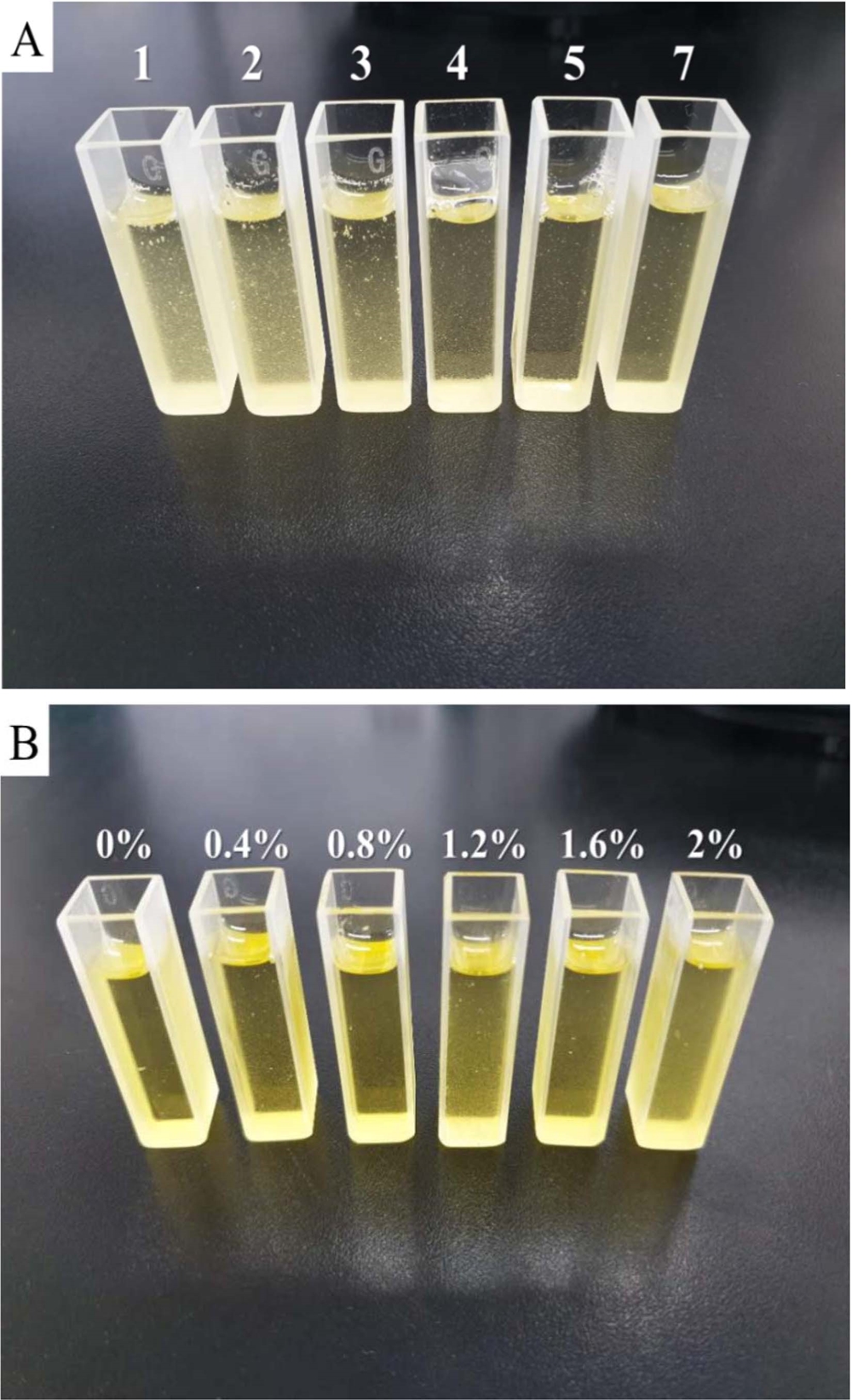
Changes in turbidity of sancho oil by sodium bicarbonate treatment.A; change in turbidity according to the standing time of sodium bicarbonate (4 g NaHCO3 treatment per 250 ㎖ of sancho oil) from the left on the day of treatment, 2, 3, 4, 5, 7 days). B; changes in turbidity depending on the concentration of sodium bicarbonate treatment (from left 0, 0.4, 0.8, 1.2, 1.6, 2.0%, respectively).
On the other hand, there was no significant difference in turbidity of sancho oil when treated with ascorbic acid. And there was no change in turbidity even in mixed treatment with sodium bicarbonate.
Antioxidants are compounds that inhibit or interfere with the oxidation process and are widely used to delay lipid oxidation. Today, questions have been raised about the safety of synthetic antioxidants, suggesting the addition of natural antioxidants to edible fats and oils.
Antioxidants are used as primary or chain breaking and secondary or prophylactic. Primary antioxidants can react with peroxyl radicals before reacting with additional unsaturated lipid molecules to convert them into more stable products. Tocopherols fall into this category. Secondary antioxidants act by other processes such as metal ionic bonding, oxygen scavenging, decomposition of hydroperoxides into non-radical products, absorption of UV radiation, and single antioxygen deactivation (Jadhav et al., 1996). Ascorbic acid is a common secondary antioxidant. Primary or a combination of the two antioxidants, primary and secondary, can have a synergistic effect.
Ascorbic acid is a natural antioxidant. Ascorbic acid can regenerate tocopherols and therefore extend their antioxidant activity (Cillard and Cillard, 1987), which is indicated by significantly lower conjugated diene values in the ascorbic acid emulsion systems after 4 days of storage (p < 0.05).
Ascorbic acid increased the antioxidant power of vegetable oil. Kartika et al. (2005) reported that the antioxidant power was increased as a result of adding ascorbic acid to sunflower oil. Ascorbic acid appears to be the most effective antioxidant according to the Rancimat oxidative stability index.
5. Stability of sodium bicarbonate against temperature
Sodium bicarbonate showed a change in acid value according to the storage temperature (Table 2). As the storage temperature increased, the acid value slightly increased. However, the acid value was significantly lower than that of sancho oil without sodium bicarbonate. However, turbidity was not significantly changed regardless of the addition of sodium bicarbonate.

Acid value and turbidity of sancho oil when sodium bicarbonate is treated at various storage temperatures.
Sodium bicarbonate is an abbreviation for sodium bicarbonate and is a colorless crystal. The solubility in 100 g of water is 8.8 g (15℃), and the aqueous solution becomes weakly alkaline because it hydrolyzes, and when heated to 65℃ or higher, CO2 is released to become sodium carbonate. It is most widely used as an antacid and baking powder. It is a white powder and has a characteristic taste. It is weakly alkaline, neutralizes stomach acid in the stomach and generates carbon dioxide gas.
The mixed treatment of sodium bicarbonate and vitamins prevented rancidity of sancho oil. vitamin C is a natural antioxidant that helps get rid of free radicals (Khassaf et al., 2003; Imik et al., 2012). Vitamin C is widely used for antioxidant, anti-aging, antioxidant stress toxicity, and is widely used by athletes to eliminate free radicals (Khassaf et al., 2003; Morrison et al., 2015; Jaiswal et al., 2017). The treatment of vitamin C in sancho oil may also be related to the tocopherol contained in sanchoi oil. Cuomo et al. (2020) reported that vitamin C added to olive oil regenerates vitamin E by vitamin C, and as a result, it was very effective that the peroxide value did not change for about 40 days after storage.
Sodium bicarbonate also maintains an acid-base and electrolyte balance (Mujahid, 2011). There are no reports of extension of the shelf life of plant oils with sodium bicarbonate. However, it has been said that sodium bicarbonate maintains the acid-base and electrolyte balance (Mujahid, 2011). Qin et al. (2017) reported that when sodium bicarbonate was treated with tartary buckwheat sprouts, the α-diphenyl-β-picrylhydrazyl (DPPH) scavenging ability increased along with the increase in bioactive substances such as flavonoids. However, the mechanism of preventing rancidity of plant oils is unknown, and further research is needed.
What is clear from our study is that it prevented rancidity of the sancho oil, thereby increasing its storage capacity. It seems that these two have created synergistic effects, such as vitamin's ability to remove excess reactive oxygen species (ROS) and sodium bicarbonate's ability to maintain acid-base and electrolyte balance.
As a result of this study, sodium bicarbonate is considered to play a very good preservative for long-term storage of sancho oil. However, in this study, the treatment period for sodium bicarbonate was only 6 months, and the change of properties due to treatment other than turbidity analysis was not investigated. Therefore, it is necessary to investigate changes in the properties of sancho oil following sodium bicarbonate treatment and long-term storage. Sodium bicarbonate is expected to be of great help in the storage and distribution of sancho oil as it has the advantage of not having any restrictions on the food code and is not harmful.
Acknowledgments
This study was carried out with the support of ´R&D Program for Forest Science Technology (Project No: 2020186A00-2022-AA02) provided by Korea Forest Service(Korea Forestry Promotion Institute).
REFFERENCES
- American Oil Chemists' Society(AOCS). (1990). AOCS official and tentative methods. (10th ed.). American Oil Chemists' Society. Urbana. IL, USA. p.30-63.
- Cha JY, Shin SR and Cho YS. (2000). Fatty acid composition of serum and liver in mice and sancho(Zanthoxylum schinifolium) seed oil. Korean Journal of Postharvest Science and Technology. 7:308-312.
- Chang KM and Kim GH. (2008). Analysis of aroma components from Zanthoxylum. Food Science and Biotechnology. 17:669-674.
-
Choochote W, Chaithong U, Kamsuk K, Jitpakdi A, Tippawangkosol P, Tuetun B, Champakaew D and Pitasawat B. (2007). Repellent activity of selected essential oils against Aedes aegypti. Fitoterapia. 78:359-364.
[https://doi.org/10.1016/j.fitote.2007.02.006]

- Chung MS. (2005). Volatile compounds of Zanthoxylum piperitum A.P. DC. Food Science and Biotechnology. 14:529-532.
- Cillard J and Cillard P. (1987). Antioxidant activity of associated alpha-tocopherol and ascorbic acid in aqueous media. Revue Française des Corps Grass. 34:271-274.
-
Cuomo F, Cinelli G, Chirascu C, Marconi E and Lopez F. (2020). Antioxidant effect of vitamins in olive oil emulsion. Colloids and Interfaces. 4:23. https://www.mdpi.com/2504-5377/4/2/23, (cited by 2020 June 10).
[https://doi.org/10.3390/colloids4020023]

-
Imik H, Ozlu H, Gumus RECEP, Atasever MA, Urcar S and Atasever M. (2012). Effects of ascorbic acid and α-lipoic acid on performance and meat quality of broilers subjected to heat stress. British Poultry Science. 53:800-808.
[https://doi.org/10.1080/00071668.2012.740615]

-
Jadhav SJ, Nimbalkar SS, Kulkarni AD and Madhavi DL. (1996). Lipid oxidation in biological and food systems. In Madhavi DL. et al. (eds.). Food Antioxidants: Technological, Toxicological, and Health Perspectives. Marcel Dekker Inc. New York. NY, USA. p.5-64.
[https://doi.org/10.1201/9781482273175]

-
Jaiswal SK, Gupta VK, Ansari MD, Siddiqi NJ and Sharma B. (2017). Vitamin C acts as a hepatoprotectant in carbofuran treated rat liver slices in vitro. Toxicology Reports. 4:265-273.
[https://doi.org/10.1016/j.toxrep.2017.06.001]

- Jeong JS and Shin MK. (1990). An unabridged dictionary of rural medicinal herbs. Youngrim Press. Seoul, Korea. p.795.
- Kang SM, Kim HG, Yang WH, Yong SH, Park DJ, Park JH, Enukwa EH and Choi MS. (2017). Changes in the physicochemical characteristics of sancho oil according to the purification process. Korean Journal of Medicinal Crop Science. 25:296-304.
-
Kartika IA, Pontalier PY and Rigal L. (2005). Oil extraction of oleic sunflower seeds by twin screw extruder: Influence of screw configuration and operating conditions. Industrial Crops and Product. 22:207-222.
[https://doi.org/10.1016/j.indcrop.2005.01.001]

-
Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA and Jackson MJ. (2003). Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. The Journal of Physiology. 549:645-652.
[https://doi.org/10.1113/jphysiol.2003.040303]

- Kim KG, Song HJ, Jeong MJ, Seo YL, Kim DI, Lim JT, Kim HG, Kang SM, Kim HJ and Choi MS. (2012). Fatty acid content and physicochemical characteristics of sancho(Zanthoxylum piperitum DC.) seed oil. Journal of Forest Genetics and Physiology. 1:1-7.
- Korea Food and Drug Administration(KFDA). (2007). Korea food code. Moonyoungsa. Seoul, Korea. p.93-100.
- Lee JH, Chang KM and Kim GH. (2009). Anti-inflammatory activities of Chopi(Zanthoxylum piperitum A. P. DC) essential oil: Suppression of the inducible nitric oxide synthase and cellular adhesion. Food Science and Biotechnology. 18:1371-1378.
- Lee JW. (1998). Volatile flavor components of Korean Sancho fruit and tree(Zanthoxylum schinfolium). Korean Journal of Food and Nutrition. 11:493-498.
-
Lee MJ, Cho MK, Oh SH, Oh CH, Choi DS, Woo JW, Park KH and Jung MY. (2014). Fatty acid composition, contents of tocopherols and phytosterols, and oxidative stability of mixed edible oil of perilla seed and rice bran oil. Korean Journal of Food and Nutrition. 27:59-65.
[https://doi.org/10.9799/ksfan.2014.27.1.059]

- Lee SJ. (1996). Korean folk medicine, monographs series No. 3. Publishing Center of Seoul National University. Seoul, Korea. p.88.
-
Morrison D, Hughes J, Della Gatta PA, Mason S, Lamon S, Russell AP and Wadley GD. (2015). Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radical Biology and Medicine. 89:852-862.
[https://doi.org/10.1016/j.freeradbiomed.2015.10.412]

-
Mujahid A. (2011). Nutritional strategies to maintain efficiency and production of chickens under high environmental temperature. The Journal of Poultry Science. 48:145-154.
[https://doi.org/10.2141/jpsa.010115]

- National Forest Seed and Variety Center(NFSVC). (2017). Report for analysis of the valuation of new forest variety development in 2017 and the ripple effect of related industries. National Forest Seed and Variety Center. Korea Forest Service. Chungju, Korea. p.89-103.
-
Park HS, Jun DY, Fang Z, Woo MH and Kim YH. (2008). Antimicrobial activity of seeds of Zanthoxylum piperitum against oral pathogen Streptococcus mutans. Journal of Life Science. 18:167-174.
[https://doi.org/10.5352/JLS.2008.18.2.167]

-
Qin P, Wei A, Zhao D, Yao Y, Yang X, Dun B and Ren G. (2017). Low concentration of sodium bicarbonate improves the bioactive compound levels and antioxidant an α-glucosidase inhibitory activities of tartary buckwheat sprouts. Food Chemistry. 224:124-130.
[https://doi.org/10.1016/j.foodchem.2016.12.059]

- Seo KL, Lee HJ and Koh KH. (1999). Antimicrobial activity of the volatile components from fruit peel of chopi(Zanthoxylum piperitum DC). Korean Journal of Applied Microbiology and Biotechnology. 27:179-183.
- Song J, Bang JK, Park HW, Park CB and Seong NS. (2003). Oxidative stability and fatty acid composition during storage of chufa oil. Journal of the Korean Society of International Agriculture. 15:31-37.