Chemical Constituents from the Aerial Parts of Vernonia cinerea L. and Their Anti-Inflammatory Activity
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Previous phytochemical studies of whole Vernonia cinerea L. plants have identified sesquiterpene lactones, sterols, and triterpenes, which possess anticancer, antifeedant, and antimalarial activities. However, there are no reports of other types of bioactive metabolites. Therefore, the present study aimed to identify phenolic compounds with anti-inflammatory activity in the aerial parts of the plant.
Compounds were isolated from the aerial parts of V. cinerea using a silica and C-18 gel columns and semipreparative HPLC instrument, and the structures of the compounds were determined using one- and two- dimension nuclear magnetic resonance spectroscopy and mass spectroscopy. The chloroform soluble extracts and isolated compounds were evaluated for their anti-inflammatory potential based on their ability to inhibit nitric oxide production and TNF-α induced NF-κB activity.
Phytochemical study of the aerial parts of V. cinerea led to the isolation of six phenolic compounds. Compound 1 was a major metabolite, and to the best of our knowledge, compounds 2 - 6 were isolated from V.cinerea for the first time. Among the isolates, compounds 1 and 3 exhibited TNF-α-induced NF-κB activity with IC50 values of 7.5 and 11.5 M, respectively, and the inhibitory activity of phenyl propanoid compound 3 on TNF-α-induced NF-κB was evaluated for the first time.
Keywords:
Vernonia cinerea L., Anti-Inflammatory Activity, Asteraceae, Phenolic CompoundsINTRODUCTION
NF-κB is a transcription factor that is associated with cell apoptosis, differentiation, and migration. Upon activation, it may promote cell proliferation and prevent cell death through anti-apoptotic factors (Baldwin, 2001). Inhibition of NF-κB signalling has a potential application for either the treatment or prevention of cancer.
Nitric oxide (NO) is an inorganic gaseous molecule that is synthesized by the oxidation of l-arginine catalyzed by nitric oxide synthase (NOS) and is involved in a number of physiological and pathological processes in mammals (Moncada et al., 1991). In the NOS family, iNOS is expressed in a variety of cells including macrophages, endothelial cells, and smooth muscle cells in response to pro-inflammatory stimuli such as IL-1β, TNF-α, and LPS. NO plays an important role in the regulation of many physiological functions, such as host defence, neurotoxicity, and vasodilation (Anggård, 1994). However, excess production of NO has been implicated in immunological and inflammatory diseases including septicshock, rheumatoid arthritis, graft rejection, and diabetes (Anggård, 1994). Therefore, inhibition of NO increase is apparently an important therapeutic consideration in the development of anti-inflammatory and cancer agents.
The leaves, roots, and seeds of some species of Vernonia (Asteraceae) have been reported to have medicinal properties (Misra et al., 1984a). Vernonia cinerea L. is an annual herb that grows in south Asia. The plant is called ‘Yaa Saam Wan’ in Thailand. It is used as a tonic, stomachic, and astringent and also known to treat consumption, asthma, and bronchitis (Kone and Kande, 2012). Aqueous ethanolic extracts showed anti-ranikhet virus and anti-cancer activities (Beeran et al., 2014). The roots are used as an anthelmintic and diuretic (Dastur, 1977). The flowers are used to treat conjunctivitis, fever, and rheumatism (Kirtikar and Basu, 1975). There have been phytochemical reports on the diverse compounds from this species, including sesquiterpene lactones, sterols, and triterpenes (Kuo et al., 2003; Chea et al., 2006; Misra et al., 1984a, b). Some of these compounds have been shown to have anticancer (Kuo et al., 2003; Pratheeshkumar and Kuttan, 2011), antimalarial (Chea et al., 2006), and antifeedant activity (Tandon et al., 1998).
In a continuing research for anti-inflammatory agents, the chloroform (CHCl3)-soluble extract of the aerial parts of V. cinerea exhibited inhibitory effects on the tumor necrosis factor alpha (TNF-α)-induced NF-κB activity and lipopolysaccharide (LPS)-induced nitric oxide (NO) production using murine macrophage RAW 264.7 cells. Although previous phytochemical studies on the whole plant of V. cinerea have reported anticancer, antimalarial, and antifeedant activity, there are no reports on other type of secondary metabolites with their anti-inflammatory activity. In particular, phenolic compounds, such as phenolic acids, flavonoids, and tannins are a large group of phytochemicals, existing ubiquitously in plants as secondary metabolites, which exhibit a wide range of biological and physiological functions, such as anti-allergenic, anti-inflammatory, antimicrobial and antioxidant activities (Balasundram et al., 2006; Manach et al., 2005; Middleton et al., 2000).
Accordingly, this research aims to find phenolic compounds with their anti-inflammatory activity, which have never been reported from the plant source. Here, we report the isolation and structure elucidation of secondary metabolites, as well as the inhibition on TNF-α-induced NF-κB activity and nitric oxide (NO) production in LPSstimulated RAW 264.7 cells.
MATERIALS AND METHODS
1. General experiments
UV spectra were recorded on a Shimadzu PharmaSpec- 1700 UV-visible spectrophotometer (Shimadzu, Kyoto, Japan). IR spectra were measured on a Bruker Tensor-27 spectrophotometer (Bruker, Billerica, MA, USA). 1D and 2D NMR spectra were recorded on a Bruker AVANCE (400 MHz) spectrometer (Bruker, Billerica, MA, USA). Low- and high-resolution mass spectrometer analyses were performed with a BioTOF II ESI mass spectrometer (Bruker, Billerica, MA, USA). Thin-layer chromatography (TLC) was performed on silica gel 60 F254 (0.25㎜, Merck, Darmstadt, Germany). Silica gel (230 - 400 mesh, Merck, Darmstadt, Germany) and C-18 (YMCGEL ODSA, 12㎚, S-150㎛, YMC Co., Ltd., Kyoto, Japan) were used for column chromatography. Semi-preparative HPLC (Beckman Coulter Inc., Brea, CA, USA) was conducted on a Beckman Coulter Gold-168 system equipped with a photodiode array detector using an Alltech reversed-phase Econosil C-18 column (10㎛, 10 × 250㎜, Alltech Inc., Nicholasville, KY, USA) with a flow rate of 2㎖/min.
2. Plant material
The aerial parts of Vernonia cinerea L. (Asteraceae) were collected from the Lampang Herb Conservation Club, Lampang Province, Thailand, in May 2011 and identified by comparison with the voucher specimen at the Forest Herbarium, Bangkok, Thailand. A voucher specimen (no. Vcw 002) was deposited at the Natural Product Chemistry Laboratory, College of Pharmacy, University of Hawaii at Hilo.
3. Extraction and isolation
The air dried aerial parts of V. cinerea (1㎏) were extracted with MeOH (2 times × 2ℓ) at room temperature. The solvent was concentrated in vacuo to yield a MeOH extract (80 g), which was then suspended in distilled water (0.5ℓ) and fractionated with chloroform (CHCl3, 2 × 1ℓ), ethyl acetate (EtOAc, 2 × 1ℓ), and nbuthanol (BuOH, 2 × 1ℓ), successively.
The CHCl3 extracts (12 g) were subjected to silica gel column chromatography (CC; ∅ 10㎝; 230 - 400 mesh, 0.5 ㎏) using a gradient solvent system of hexane-ethyl acetate (100 : 0 to 30 : 70), to afford 57 fractions (C1- C57). The fractions (1 g) combined from C54 to C57 were subjected to silica gel CC (∅ 8㎝; 230 - 400 mesh, 80 g), with hexane-ethyl acetate (100 : 0 to 1 : 1) as the solvent system, yielding seven subfractions (C54S1 to C54S7). Subfraction C54S4 (0.5 g) was chromatographed on a sephadex LH-20 gel (50 g) column and eluted with H2O-MeOH (100 : 0 to 50 : 50), to afford five subfractions (C54S4L1 to C54S4L5), and compound 3 (1.5㎎, 0.000019% recovery from the extract) was recrystallized from subfraction C54S4L2. Subfraction C54S4L3 (0.1 g) was subjected to preparative. HPLC (MeOH-H2O=40 : 60 to 100 : 0) to yield 6 (5㎎, tR: 98 min, 0.00005%), 5 (2㎎, tR: 105 min, 0.000025%), 4 (1㎎, tR: 108 min, 0.000012%), and 2 (4㎎, tR: 115 min, 0.00005%).
Quercetin 3-O-β-D-glucopyranoside, 1 (30㎎, 0.00038%) was recrystallized in a solvent mixture, (CHCl3: MeOH, 1 : 1) from the ethyl acetate portion.
- 1) Quercetin 3-O-β-D-glucopyranoside - Yellow amorphous powder, UV (MeOH) λmax (log ε) 279 (4.0), 330 (3.7) ㎚; IR νmax (KBr) 3325, 1655㎝-1; ESI-MS m/z 465 [M+ H]+; 1H NMR (400 MHz, DMSO-d6) δH 7.48 (1H, dd, J = 8.0, 2.0 Hz, H-6'), 7.46 (1H, d, J = 8.0 Hz, H- 5'), 6.94 (1H, d, J = 2.0 Hz, H-2'), 6.82 (1H, d, J = 2.0Hz, H-8), 6.48 (1H, d, J = 2.0 Hz, H-6), 5.12 (1H, d, J = 7.2 Hz, H-1"), 3.75 (1H, d, J = 10.4 Hz, H-6a"), 3.53 (1H, d, J = 10.4 Hz, H-6b"), 3.20-3.53 (4H, m, H-2" to H- 5"), 12.96 (1H, s, OH-5); 13C NMR (100 MHz, DMSOd6) δc 182.3 (C-4), 164.9 (C-7), 163.4 (C-5), 161.6 (C-9), 157.4 (C-2), 150.4 (C-4'), 146.2 (C-3'), 121.8 (C-1'), 119.6 (C-6'), 116.6 (C-5'), 114.0 (C-2'), 105.8 (C-10), 103.6 (C- 1"), 100.3 (C-6), 95.1 (C-8), 77.6 (C-3"), 76.8 (C-5"), 73.5 (C-2"), 70.0 (C-4"), 61.0 (C-6").
- 2) (E)-4-(3,4-Dimethoxyphenyl)but-3-en-1-ol - Yellow amorphous powder, UV (MeOH) λmax (log ) 280 (4.1) ㎚; ESI-MS m/z 209 [M + H]+; 1H NMR (400MHz, CD3OD) δH 6.94 (1H, d, J = 2.0 Hz, H-2'), 6.91 (1H, dd, J = 7.4, 2.0 Hz, H-6'), 6.82 (1H, d, J = 7.4 Hz, H-5'), 6.44 (1H, d, J = 16.4 Hz, H-4), 6.09 (1H, dt, J = 16.4, 6.8 Hz, H-3), 3.77 (1H, t, J = 6.6 Hz, H-1), 2.50 (1H, q, J = 6.6 Hz, H- 2), 3.92 (3H, s, 3'-OCH3), 3.89 (3H, s, 4'-OCH3); 13C NMR (100 MHz, CD3OD) δc 149.1 (C-4'), 148.8 (C-3'), 132.1 (C-4), 130.5 (C-1'), 124.2 (C-6'), 124.2 (C-3), 111.3 (C-5'), 108.8 (C-2'), 62.0 (C-1), 36.1 (C-2), 55.7 (4'- OCH3), 55.6 (3'-OCH3).
- 3) 3-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-propan-1- one - White amorphous powder, UV (MeOH) λmax (log ε) 254 (3.6) ㎚; ESI-MS m/z 197 [M + H]+; 1H NMR (400 MHz, CD3OD) δH 7.57 (1H, dd, J = 8.4, 2.0 Hz, H- 6'), 6.98 (1H, d, J = 8.4 Hz, H-5'), 6.93 (1H, d, J = 2.0 Hz, H-2'), 4.04 (2H, t, J = 5.2 Hz, H-3), 3.98 (3H, s, 3'-OMe), 3.20 (2H, t, J = 5.2 Hz, H-2); 13C NMR (100 MHz, CD3OD) δc 199.0 (C-1), 151.0 (C-4'), 147.4 (C-3'), 131.2 (C-1'), 124.4 (C-6'), 115.8 (C-2'), 112.1 (C- 5'), 59.0 (C-9'), 42.0 (C-8'), 56.7 (3'-OMe).
- 4) 1H-Indole-3-carbaldehyde - White amorphous powder, ESI-MS m/z 146 [M+ H]+; 1H NMR (400 MHz, CD3OD) δH 9.90 (1H, s, CHO), 8.85 (1H, dd, J = 6.8, 1.2 Hz, H-4), 8.17 (1H, s, H-2), 7.48 (1H, dd, J = 6.8, 1.2 Hz, H-7), 7.28 (2H, m, H-5/H-6); 13C NMR (100 MHz, CD3OD) δc 185.5 (CHO), 138.0 (C-2), 137.0 (C- 1a), 124.3 (C-6), 124.0 (C-3a), 122.5 (C-5), 121.5 (C-4), 117.0 (C-3), 112.2 (C-7).
- 5) trans-Cinnamic acid - Yellow amorphous powder, UV (MeOH) λmax (log ) 280 (4.0) ㎚; ESI-MS m/z 149 [M + H]+; 1H NMR (400MHz, CD3OD) δH 7.72 (1H, d, J = 16.0 Hz, H-3), 7.60 (2H, m, H-2'/H-6'), 7.42 (3H, m, H-3'/H-4'/H-5'), 6.50 (1H, d, J = 16.0 Hz, H-2); 13C NMR (100 MHz, CD3OD) δc 172.0 (C-1), 147.9 (C-3), 115.3 (C-3), 134.2 (C-1'), 129.3 (C-3'/C-5'), 125.5 (C-2'/C-6'), 127.4 (C-4').
- 6) Uracil - White amorphous powder, ESI-MS m/z 113 [M + H]+; 1H NMR (400MHz, DMSO-d6) δH 5.45 (1H, d, J = 7.6 Hz, H-5), 7.39 (1H, d, J = 7.6 Hz, H-6); 13C NMR (100 MHz, DMSO-d6) δc 164.7 (C-4), 151.7 (C-2), 142.5 (C-6), 100.4 (C-5).
4. Tumor necrosis factor-α (TNF-α) activated nuclear factor-kappa B (NF-κB) assay
Human embryonic kidney cells 293 Panomics (Fremont, CA, USA) were employed for monitoring changes occurring along the NF-κB pathway (Kondratyuk et al., 2012). Stable constructed cells were seeded into 96-well plates at 20 × 103 cells per well. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen Co., Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS), 100 units/㎖ penicillin, 100㎍/㎖ streptomycin, and 2 mM l-glutamine. After 48 h incubation, the medium was replaced and the cells were treated with various concentrations of test substances. TNF-α (Human, Recombinant, E. coli, Calbiochem, San Diego, CA, USA) was used as an activator at a concentration of 2 ng/㎖ (0.14 nM). The plate was incubated for 6 h. Spent medium was discarded and the cells were washed once with PBS. Cells were lysed using 50㎕ (for 96-well plate) reporter lysis buffer from Promega, by incubating for 5 min on a shaker, and stored at −80℃. The luciferase assay was performed using the Luc assay system from Promega (Promega Co., Madison, WI, USA). The gene product, luciferase enzyme, reacts with luciferase substrate, emitting light which was detected using a luminometer (LUMIstar Galaxy BMG, Ortenberg, Germany). Data for NF-κB constructs are expressed as IC50 values (i.e. concentration required to inhibit TNF-activated NF-κB activity by 50%). As a positive control, two known NF-κB inhibitors were used: TPCK, IC50= 3.8 μM and BAY-11, IC50= 2.0 μM.
5. Inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells (iNOp) assay
The level of nitrite, the stable end product of NO, was estimated as described previously (Park et al., 2011). Briefly, RAW 264.7 cells were seeded and incubated in 96-well culture plates at 37℃, 5% CO2 in a humidified air for 24 h. The cultured medium was replaced with phenol red-free medium containing various concentrations of compounds for 15 min prior to 1㎎/㎖ of LPS exposure for 20 h. The amount of nitrite in the culture media was measured by using Griess reagent. Under the same experimental conditions, SRB assays were performed to evaluate the cytotoxic effect of compounds toward RAW 264.7 cells. L-NG-monomethyl arginine citrate (l- NMMA), as a positive control of this assay showed an IC50 value of 25.1 μM.
6. Statistical analysis
All data were expressed as means ± SD. Statistical analysis was performed using SPSS software (version 18.0, SPSS Inc., Chicago, IL, USA). Significant differences between the groups were determined using the analysis of variance (ANOVA) followed by Duncan Post Hoc Test (DPHT). p < 0.05 were considered as statistical significance.
RESULTS AND DISCUSSION
1. Structure elucidation of compounds 1 - 6
Repeated chromatography of the aerial parts of V. cinerea on silica gel and YMC-pack RP-C18 columns led to the isolation of six known compounds (1 - 6) (Fig. 1).
Compound 1 was obtained as a light yellow amorphous powder and its molecular weight was evaluated as m/z 465 [M+ H]+ by the positive ESI-MS spectrometry. The UV spectrum showed absorption maxima at 330 and 279㎚ indicating a conjugated double bond and a carbonyl group, respectively. The IR spectrum displayed characteristic absorption bands at 3,325 and 1,655㎝−1, corresponding to the hydroxy group (s) and conjugated C = O group (s), respectively. The 13C NMR spectrum revealed 15 carbon signals, along with six glucose unit carbons at [δc 103.6 (C-1"), 77.6 (C-3"), 76.8 (C-5"), 73.5 (C-2"), 70.0 (C-4"), 61.0 (C-6")], suggested that 1 is a glycosylated flavone skeleton (Han et al., 2004). The 1H NMR spectrum showed a downfield shifted OH proton at δH 12.96 (1H, s, OH-5) due to the hydrogen bond with a carbonyl group (C-4), and two aromatic doublet protons at δH 6.82 (1H, d, J = 2.0 Hz, H-8) and 6.48 (1H, d, J = 2.0 Hz, H-6), suggesting a di-substituted A-ring. In addition, the 1H NMR spectrum displayed ABX type aromatic system protons at [δH 7.48 (1H, dd, J = 8.0, 2.0 Hz, H-6'), 7.46 (1H, d, J = 8.0 Hz, H-5'), and 6.94 (1H, d, J = 2.0 Hz, H-2')] in B-ring and glycose unit protons at [δH 5.12 (1H, d, J = 7.2 Hz, H-1"), 3.75 (1H, d, J = 10.4 Hz, H-6"a), 3.53 (1H, d, J = 10.4 Hz, H-6"b), 3.20 - 3.53 (4H, m, H-2" to H- 5")]. The heteronuclear multiple bond correlation (HMBC) correlation of the anomeric proton (H-1") with C-3 indicated the position of glycose unit at C-3. Accordingly, compound 1 was assigned as quercetin 3-O-β-D-glucopyranoside (1), by comparison of its physicochemical data with literature values (Choi et al., 2000).
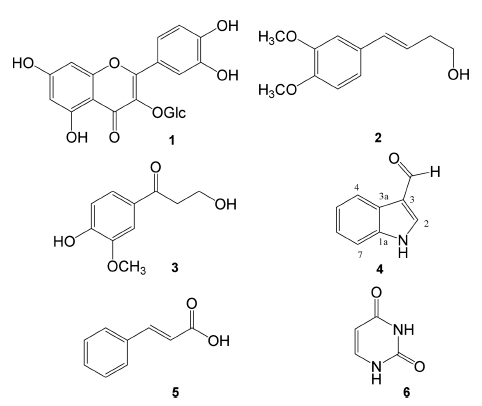
Compound 2 was obtained as a yellow amorphous powder. The ESI-MS spectrum of 2 gave a molecular ion [M + H] at m/z 209. The NMR and heteronuclear singlequantum correlation (HSQC) spectra of 2 displayed ABXtype aromatic protons at [δH 6.94 (1H, d, J = 2.0 Hz, H- 2'), 6.91 (1H, dd, J = 7.4, 2.0 Hz, H-6'), and 6.82 (1H, d, J = 7.4 Hz, H-5')] with six aromatic carbons at [δc 149.1 (C-4'), 148.8 (C-3'), 130.5 (C-1'), 124.2 (C-6'), 111.3 (C- 5'), and 108.8 (C-2')], a trans-olefinic group at δH 6.44 (1H, d, J = 16.4 Hz)/δc 132.1 (C-4) and at 6.09 (1H, dt, J = 16.4, 6.8 Hz/δc 124.2 (C-3), and two methoxy groups at δH 3.92/δc 55.6 (3'-OCH3) and at δH 3.89/δc 55.7 (4'- OCH3). The position of two methoxy signals at (δH 3.92 and 3.89) was confirmed at C-3' and C-4', respectively, through the HMBC analysis (Fig. 2). The remaining methylene at δH 2.50 (1H, q, J = 6.6 Hz)/δc 36.1 (C-2) and an oxygen bearing methylene group at δH 3.77 (1H, t, J = 6.6Hz)/δc 62.0 (C-1) were confirmed as a part of the trans-olefinic group, on the basis of the HMBC correlations from H-1 to C-3 and from H-2 to C-4. In addition, three-bond HMBC correlations of the olefinic proton (H-4) with C-2'/C-6' and of H-2'/H-6' with C-4 were shown as in Fig. 2, indicating a but-3-en-1-ol group was attached at C-1' of the phenyl group. Accordingly, the structure of 2 was identified as (E)-4-(3,4-dimethoxyphenyl)but- 3-en-1-ol (Masuda and Jitoe, 1995).
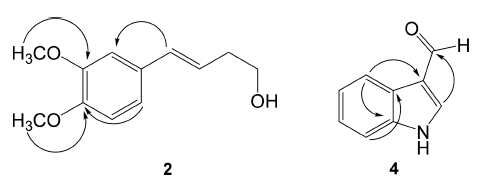
Compound 4 was obtained as a white amorphous powder. The MS spectrum showed a molecular ion peak at m/z 146 [M+ H]+. The NMR and HSQC spectra of 4 displayed signals for a tri-substituted olefinic group at δH 8.17 (1H, s)/δc (138.0, C-2) and δc (117.0, C-3), an aromatic quaternary carbon at δc 124.0 (C-3a), a downfield shifted aromatic quaternary at δc 137.0 (C-1a) due to a nitrogen atom attachment, and four protonated aromatic signals at [δH 8.85 (1H, dd, J = 6.8, 1.2 Hz)/δc 121.5 (C-4), 8.17 (1H, s)/δc 138.0 (C-2), 7.48 (1H, dd, J = 6.8, 1.2 Hz)/δc 112.2 (C-7), δh 7.28 (1H, m)/δc 122.5 (C-5), and δh 7.28 (1H, m)/δc 124.3 (C-6)], indicated the presence of an indole skeleton (Wang et al., 2013). In addition, the NMR spectra revealed an aldehyde group signal at δH 9.90 (1H, s)/δc 185.5 (CHO). The HMBC spectrum displayed two- to three-bonds correlations from H-2 to aldehyde carbon (CHO)/C-3a and from H-4 to C-3/ C-1a/C-3a (Fig. 2), confirmed the identification of compound 4 as 1H-indole-3-carbaldehyde, previously reported from Laminaria japonica (Wang et al., 2013).
The other isolates were identified as, 3-hydroxy-1-(4- hydroxy-3-methoxyphenyl)-propan-1-one (3) (Jones et al., 2000), trans-cinnamic acid (5) (Davidse et al., 1990), and uracil (6) (Kitajima et al., 1999), by comparison of their physical and spectral data with published values. Among the isolates, compound 1 was obtained as a major metabolite. To the best of our knowledge, compounds 2 - 6 were isolated for the first time from this plant source.
2. Evaluation of anti-inflammatory activity
The CHCl3-soluble portion from the aerial parts of V. cinerea exhibited potent inhibitory activity against TNF-α- induced NF-κB activity and NO production in LPSstimulated RAW 264.7 cells with inhibition rates of 62.3 and 63.3%, respectively. Compounds 1 - 3 isolated from the CHCl3 extract were also evaluated for their antiinflammatory potential based on their ability to inhibit TNF-α-induced NF-κB activity and NO production, while compounds 4 - 6 could not be evaluated in this manner, due to their insufficient amounts available for testing. As shown in Table 1, when treated with a fixed concentration of 50 μM, quercetin glycoside, 1 and phenyl propanoid, 3 exhibited moderate TNF-α-induced NF-κB inhibitory activity with IC50 values of 7.5 and 11.5 μM, respectively, compare to that of the positive control, tosyl phenylalanyl chloromethyl ketone (TPCK, IC50= 3.8M), whereas compounds 1 - 3 showed weak inhibitory activity on NO production.

Inhibition effect of compounds 1 - 3 on the TNF-α-induced NF-κB activity and NO production in LPS-stimulated RAW 264.7 cells.
Compound 3 has been reported to have weak activity on NO production (Min and Cuong, 2013), but inhibitory activity of 3 on TNF-α-induced NF-κB was evaluated first time in this study. In addition, quercetin and its derivatives have been found to possess NF-κB inhibitory and antioxidant effects (Kim et al., 2013; Lee et al., 2013).
In a previous research for cancer chemopreventive agents, major secondary metabolites, sesquiterpene lactones isolated from the flower of V. cinerea exhibited potent inhibitory effects on the tumor necrosis factor alpha (TNF- α)-induced NF-κB activity and lipopolysaccharide (LPS)- induced nitric oxide production using murine macrophage RAW 264.7 cells (Youn et al., 2012). Although the sesquiterpene lactones have been reported to have a potential on the cancer chemopreventive activity in the previous study, the anti-inflammatory activity of several phenolic compounds isolated from the aerial parts of V. cinerea can be attributed, at least in part, to inhibition of TNF-α-induced NF-κB activity.
ACKNOWLEDGMENTS
This study was supported by a grant to the Korea Polar Research Institute (KOPRI), under a project PE16350. We thank H. S. Shin, National center for inter University Research Facilities, Seoul National University, for the provision of the Mass Spectrometry Facility used in this study.
References
- Anggård, E, Nitric oxide Mediator, murderer, and medicine, Lancet, (1994), 343, p1199-1206.
-
Baldwin, AS, Control of oncogenesis and cancer therapy resistance by the transcription factor NF-?B, Journal of Clinical Investigation, (2001), 107, p241-246.
[https://doi.org/10.1172/jci11991]

- Beeran, AA, Maliyakkal, N, Rao, CM, Udupa, N, The enriched fraction of Vernonia cinerea L induces apoptosis and inhibits multi-drug resistance transporters in human epithelial cancer cells, Journal of Ethnopharmacology, (2014), 158, p33-42.
-
Balasundram, N, Sundram, K, Samman, S, Phenolic compounds in plants and agri-industrial by-products Antioxidant activity, occurrence, and potential uses, Food Chemistry, (2006), 99, p191-203.
[https://doi.org/10.1016/j.foodchem.2005.07.042]

-
Chea, A, Hout, S, Long, C, Marcourt, L, Faure, R, Azas, N, Elias, R, Antimalarial activity of sesquiterpene lactones from Vernonia cinerea, Chemical and Pharmaceutical Bulletin, (2006), 54, p1437-1439.
[https://doi.org/10.1248/cpb.54.1437]

- Choi, YH, Kim, JH, Kim, MJ, Han, SS, Rim, YS, Antioxidative compounds in leaves of Castanea crenata S Et Z, Korean Journal of Medicinal Crop Sciences, (2000), 8, p373-377.
- Dastur, JF, Medicinal plants of India and Pakistan, (1977), D.B Taraporevala Sons and Co., Ltd, Maharashtra, India, p174.
-
Davidse, PA, Dillen, JLM, Heyns, AM, Modro, TA, Rooyen, PHV, Photochromic systems. Part 1. Structural and spectroscopic study of photochromically active products of Stobbe condensation 2,3-Dibenzylidenesuccinic acid and its anhydride, Canadian Journal of Chemistry, (1990), 68, p741-746.
[https://doi.org/10.1139/v90-117]

-
Han, JT, Bang, MH, Chun, OK, Kim, DO, Lee, CY, Baek, NI, Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities, Archives of Pharmacal Research, (2004), 27, p390-395.
[https://doi.org/10.1007/bf02980079]

-
Jones, L, Bartholomew, B, Latif, Z, Sarker, SD, Nash, RJ, Constituents of Cassia laevigata, Fitoterapia, (2000), 71, p580-583.
[https://doi.org/10.1016/s0367-326x(00)00155-6]

-
Kim, BH, Choi, JS, Yi, EH, Lee, JK, Won, C, Ye, SK, Kim, MH, Relative antioxidant activities of quercetin and its structurally related substances and their effects on NF-?B/ CRE/AP-1 signaling in murine macrophages, Molecules and Cells, (2013), 35, p410-420.
[https://doi.org/10.1007/s10059-013-0031-z]

- Kirtikar, KR, Basu, BD, an, I C S, Indian medicinal plants. Bishen Singh Mahendra Pal Singh, (1975), Dehradun, India, p1322.
- Kitajima, J, Ishikawa, T, Tanaka, T, Ida, Y, Watersoluble constituents of fennel IX Glucides and nucleosides, Chemical and Pharmaceutical Bulletin, (1999), 47, p988-992.
-
Kondratyuk, TP, Park, EJ, Yu, R, van Breemen, RB, Asolkar, RN, Murphy, BT, Fenical, W, Pezzuto, JM, Novel marine phenazines as potential cancer chemopreventive and anti-inflammatory agents, Marine Drugs, (2012), 10, p451-464.
[https://doi.org/10.3390/md10020451]

-
Kone, WM, Kande, B, Qualitative analysis of the pyrrolizidine alkaloids from 11 Asteraceae and Boraginaceae used in traditional medicine in Cote d'Ivoire, Research Journal of Phytochemistry, (2012), 6, p75-83.
[https://doi.org/10.3923/rjphyto.2012.75.83]

-
Kuo, YH, Kuo, YJ, Yu, AS, Wu, MD, Ong, CW, Kuo, LMY, Huang, JT, Chen, CF, Li, SY, Two novel sesquiterpene lactones, cytotoxic vernolide-A and -B, from Vernonia cinerea, Chemical and Pharmaceutical Bulletin, (2003), 51, p425-426.
[https://doi.org/10.1248/cpb.51.425]

-
Lee, CS, Jeong, EB, Kim, YJ, Lee, MS, Seo, SJ, Park, KH, Lee, MW, Quercetin-3-O-(2''-galloyl)-α-L-rhamnopyranoside inhibits TNF-α-activated NF-κB-induced inflammatory mediator production by suppressing ERK activation, International Immunopharmacology, (2013), 16, p481-487.
[https://doi.org/10.1016/j.intimp.2013.05.001]

-
Manach, C, Mazur, A, Scalbert, A, Polyphenols and prevention of cardiovascular diseases, Current Opinion in Lipidology, (2005), 16, p77-84.
[https://doi.org/10.1097/00041433-200502000-00013]

-
Masuda, T, Jitoe, A, Phenylbutenoid monomers from the rhizomes of Zingiber cassumunar, Phytochemistry, (1995), 39, p459-461.
[https://doi.org/10.1016/0031-9422(94)00883-u]

- Middleton, E Jr, Kandaswami, C, Theoharis, TC, The effects of plant flavonoids on mammalian cells Implications for inflammation, heart disease, and cancer, Pharmacological Reviews, (2000), 52, p673-751.
- Min, BS, Cuong, TD, Phenolic compounds from Caesalpinia sappan and their inhibitory effects on LPS-induced NO production in RAW 264.7 cells, Natural Product Sciences, (2013), 19, p201-205.
- Misra, TN, Singh, RS, Upadhyay, J, Srivastava, R, Chemical constituents of Vernonia cinerea, part I Isolation and spectral studies of triterpenes, Journal of Natural Products, (1984a), 47, p368-372.
- Misra, TN, Singh, RS, Upadhyay, J, Srivastava, R, Isolation of a natural sterol and an aliphatic acid from Vernonia cinerea, Phytochemistry, (1984b), 23, p415-417.
- Moncada SRMJ, Palmer RML, Higgs, EA, Nitric oxide Physiology, pathophysiology, and pharmacology, Pharmacological Reviews, (1991), 43, p109-142.
-
Park, EJ, Kondratyuk, TP, Morrell, A, Kiselev, E, Conda- Sheridan, M, Cushman, M, Ahn, S, Choi, Y, White, JJ, vanBreemen, RB, Pezzuto, JM, Induction of retinoid X receptor activity and consequent upregulation of p21WAF1/CIP1 by indenoisoquinolines in MCF7 cells, Cancer Prevention Research, (2011), 4, p592-607.
[https://doi.org/10.1158/1940-6207.capr-10-0004]

- Pratheeshkumar, P, Kuttan, G, Effect of vernolide-A, a sesquiterpene lactone from Vernonia cinerea L on cellmediated immune response in B16F-10 metastatic melanomabearing mice, Immunopharmacology and Immunotoxicology, (2011), 33, p533-538.
-
Tandon, M, Shukla, YN, Tripathi, AK, Singh, SC, Insect antifeedant principles from Vernonia cinerea, Phytotherapy Research, (1998), 12, p195-199.
[https://doi.org/10.1002/(sici)1099-1573(199805)12:3<195::aid-ptr223>3.0.co;2-#]

-
Wang, C, Yang, Y, Mei, Z, Yang, X, Cytotoxic compounds from Laminaria japonica, Chemistry of Natural Compounds, (2013), 49, p699-701.
[https://doi.org/10.1007/s10600-013-0711-0]

-
Youn, UJ, Park, EJ, Kondratyuk, TP, Simmons, CJ, Borris, RP, Tanamatayarat, P, Wongwiwatthananukit, S, Toyama, O, ongsak, T, Pezzuto, JM, Chang, LC, Antiinflammatory sesquiterpene lactones from the flower of Vernonia cinerea, Bioorganic and Medicinal Chemistry Letters, (2012), 22, p5559-5562.
[https://doi.org/10.1016/j.bmcl.2012.07.010]