
Effects of 6-Shogaol, A Major Component of Zingiber officinale Roscoe, on Human Cytochrome P450 Enzymes in vitro
© The Korean Society of Medicinal Crop Science. All rights reserved
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Ginger has been extensively used in foods and traditional medicines in Asian countries. Despite its frequent consumption in daily life, the mechanism of potential interactions between ginger components-drug has not been examined. To elucidate the mechanism of governing the effects of 6-shogaol, a primary constituent of dried ginger, on human cytochrome P450 (CYP) isoenzymes an incubation studies were carried out using pooled human liver microsome (HLM).
CYP isoenzyme specific substrate was incubated with multiple concentrations of inhibitor, HLM and cofactors. 6-shogaol showed a potent inhibitory effect on CYP2C9, CYP1A2 and CYP2C19 with half maximal inhibitory concentration (IC50) values of 29.20, 20.68 and 18.78 μM respectively. To estimate the value of the inhibition constant (Ki) and the mode of inhibition, an incubation study with varying concentrations of each CYP isoenzyme-specific probe was performed. 6-shogaol inhibited CYP2C9 and CYP2C19 noncompetitively (Ki = 29.02 and 19.26 μM respectively), in contrast, the inhibition of CYP1A2 was best explained by competitive inhibition (Ki = 6.33 μM).
These findings suggest that 6-shogaol may possess inhibitory effects on metabolic activities mediated by CYP1A2, CYP2C9 and CYP2C19 in humans.
Keywords:
Zingiber officinale, Cytochrome P450, Inhibition Constant, 6-ShogaolINTRODUCTION
Ginger (the rhizome of the Zingiber officinale Roscoe) has been commonly used as a spice in food and beverage and also as a traditional herbal medicine taken for a variety of clinical disorders such as nausea and vomiting in many Asian countries (Ernst and Pittler, 2000). Ginger contain volatile oil (~ 3%) and nonvolatile pungent components. Among the ginger oleoresin, gingerols and shogaols were identified as active ingredients. Gingerols are compounds with various unbranched alkyl chain length, while shogaols have the dehydrated chain at C4 and C5. It is well known that the amount of shogaols is increased during longterm storage and thermal processing (Yu et al., 2011). Ginger has received wide attention because of its potent antioxidant and anti-inflammatory activities (Tsai et al., 2005). In addition, a number of experiments have shown that ginger possesses cancer preventive properties and antineoplastic effects (Baliga et al., 2011; Shukla and Singh, 2007). These properties of ginger are attributed to its major pungent components such as gingerols, shogaols. 6-shogaol have gained great interest because of recent studies demonstrating its higher anticancer properties over gingerol. An experiment revealed that 6-shogaol exhibited a strong inhibition of the growth of A2780 ovarian cancer cell, while 6, 8, 10-gingerols had no effect (Rhode et al., 2007). It is also reported that 6-shogaol showed a stronger growth inhibition on SKOV-3 human ovarian cancer cell, A549 human lung cancer cell, SK-MEL-2 human skin cancer cell than 4, 6, 8, 10-gingerols (Kim et al., 2008). Some researches suggested that 6-shogaol would be a therapeutic agent for lipopolysaccharide induced neurodegenerative disorders because of its anti-inflammatory and anti-apoptotic properties in neuronal cells (Shim et al., 2011, 2012). A recent study indicated that 6-shogaol demonstrated more potent antioxidant activity than 6, 8, 10-gingerols (Dugasani et al., 2010). The liver encompasses microsome hemoproteins called cytochrome P450 which play a major role in the metabolic monooxygenation of various endogenous and exogenous compounds. Of the cytochrome P450 (CYP) family, CYP3A4 is the most abundant hemoprotein and represents about 30% of the total CYP enzymes in human liver. The second abundant CYP protein is CYP2C subfamily, representing about 25% and CYP1A2, CYP2D6 and CYP2E1 represent 10, 5 and 10%, respectively (Pelkonen and Breimer, 1994). With the popularity of herbal medicinal products, herb drug interactions have been major issues in the last decade, especially such as the interactions of St. John’s wort prescribed medicines via cytochrome P450 induction (Izzo and Ernst, 2009). In case of ginger, despite its widespread use, little examination of herb drug interactions with main components of ginger have been carried out and reported to date. According to the investigation of effects of 6-gingerol on cytochrome P450 in human liver microsome by Joo and Lim (2011), 6-gingerol demonstrated inhibitory effects on several CYP isoenzymes, especially CYP2D6, CYP2E1, CYP1A2 and CYP2C19. But the effects of 6-shogaol, a primary constituent of dried ginger, on human cytochrome P450 has not been identified yet. The aim of this study was to investigate the influence of 6-shogaol on human cytochrome P450 enzymes which are responsible for phase I metabolism of most drugs with human liver microsome and elucidate the mode of inhibition.
MATERIALS AND METHODS
1. Chemicals
6-shogaol was purchased from Wako pure chemical industries (Osaka, Japan). Acetaminophen, chlorpropamide, tolbutamide, chlorzoxazone, phenacetin, carbamazepine, 1- hydroxymidazolam, 6-hydroxychlorzoxazone, DL-propranolol HCl, (+/–)-4-hydroxymephenytoin and hydroxytolbutamide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Midazolam was bought from Toronto research chemicals (North York, ON, Canada). S-mephenytoin was purchased from BD Biosciences (San Jose, CA, USA). All other chemicals and reagents used were of analytical and HPLC grade.
2. Enzymes
Pooled human liver microsomes and NADPH regenerating systems were purchased from BD Biosciences (San Jose, CA, USA). Pooled human liver microsomes 20mg/mℓ were diluted to 5mg/mℓ with dilution buffer upon arrival and transferred to microtubes. Aliquots were kept at 80°C until they were used.
3. Enzyme assays
The inhibitory effect of 6-shogaol on the activity of each CYP isoenzyme was tested with pooled human liver microsome. CYP isoenzyme specific reactions used in this study were as follows: Midazolam 1-hydroxylation for CYP3A4 (Wang et al., 2005), phenacetin O-deethylation for CYP1A2 (Tassaneeyakul et al., 1993), tolbutamide 4- methylhydroxylation for CYP2C9 (Relling et al., 1990), Smephenytoin 4'-hydroxylation for CYP2C19 (Wrighton et al., 1993) and chlorzoxazone 6-hydroxylation for CYP2E1 (Court et al., 1997). Before the inhibition study of 6- shogaol, experiments for estimating of apparent kinetic parameters (Vmax and Km) of each CYP isoenzyme substrate were performed according to each CYP isoenzyme specific reaction with various concentrations of each CYP isoenzyme corresponding substrate, human liver microsome and NADPH regenerating system. Each substrate was incubated for a specific time interval determined by each CYP protocol and after reaction, the formed metabolite was analyzed using HPLC. Metabolite concentrations were calculated by interpolating the ratio of metabolite area to internal standard area from a calibration curve of known metabolite concentrations. Based on the calculated Km of each CYP isoenzyme substrate, the concentration of each CYP isoenzyme substrate was determined close to its Km value or a value within linear range for velocity of each CYP isoenzyme specific metabolite formation: 2 μM midazolam, 100 μM phenacetin, 50μM chlorzoxazone, 100 μM S-mephenytoin and 100 μM tolbutamide. The inhibition study of 6-shogaol on the activity of each CYP isoenzyme was carried out as follows. Each CYP isoenzyme substrate concentration and multiple concentration of 6- shogaol were added to reaction tube. Before incubation, to avoid the effect of organic solvent on the inhibition study, organic solvent used for dissolving each CYP substrate and 6-shogaol was entirely removed using a rotary evaporator. Following the identification of the total removal of organic solvent, phosphate buffer saline (PBS, pH 7.4) and distilled water were added to each reaction tube. After strong vortexing, human liver microsome was added to each reaction tube. All incubation mixtures including CYP isoenzyme specific substrate, PBS (pH 7.4), human liver microsome and various concentrations of inhibitor or without inhibitor were prewarmed for 5 min in a 37°C shaking water bath. After prewarming, CYP isoenzyme specific probe reactions were initiated by the addition of a NADPH regenerating system [1.3mM NADP+, 3.3mM glucose-6-phosphate (G6P), 1.0 U/mℓ glucose-6-phosphate dehydrogenase (G6PDH) and 3.3mM MgCl2]. After further incubation for each CYP isoenzyme specific time in a 37°C shaking water bath, the reaction was terminated by placing tubes on the ice and adding ice cold acetonitrile with each CYP isoenzyme specific internal standard. After centrifugation (14,000 rpm, 5min, 4°C), aliquots of supernatants were injected onto the HPLC system for analysis. For estimation of the inhibition constant (Ki) and the inhibition type of each CYP isoenzyme which showed an enzyme activity suppressed by 6-shogaol, a mixture which consists of substrate at multiple concentrations, multiple concentrations of inhibitor or without inhibitor, human liver microsome and cofactors was incubated according to CYP isoenzyme reaction procedure described in detail above and analyzed by HPLC.
4. Analytical procedures
HPLC system was used for measurement of metabolites and internal standard specific for CYP isoenzymes. The HPLC system consisted of a Shimadzu (Kyoto, Japan) model LC-20AD pump, a model CTO20A column oven, a model SIL-20AC autosampler and a model SPD-20A UV/ VIS detector. The concentration of metabolite was calculated with the ratio of metabolite area to internal standard area used for each specific CYP isoenzyme. Methodological details for each CYP isoenzyme assay are listed in Table 1.
5. Data analysis
IC50 value (inhibitor concentration required to cause 50% inhibition of the original enzyme activity) was determined by analysis of plot of inhibitor concentrations versus percentage of enzyme activity with Sigmaplot program (ver. 9.0, Systat Software, Chicago, IL, USA). Apparent kinetic parameters (Km, Vmax) for each CYP were calculated by fitting the plot of substrate concentration versus velocity of metabolite formation using Sigmaplot program (ver. 9.0, Systat Software, Chicago, IL, USA). To identify the type of inhibition model and calculate Ki values (apparent inhibitory constant), 3 different enzyme kinetic models (competitive, noncompetitive, uncompetitive inhibition model) were used to fit the inhibition data with WinNonlin program (ver. 5.2, Pharsight Co., Cary, NC, USA). Based on visual inspection of Lineweaver-Burk plot and Dixon plot, Akaike information criterion values and Schwartz criterion values, the most appropriate inhibition model was selected.
RESULTS
To investigate the inhibitory effect of 6-shogaol on each CYP isoenzyme, reactions with multiple concentrations of 6-shogaol (1 - 50 μM) or without 6-shogaol (control) were conducted using CYP isoenzyme specific substrate probes of which concentrations were close to their respective Km values or values within the linear range for reaction velocity. As shown in Fig. 1, 6-shogaol showed a potent inhibitory effect on CYP1A2, CYP2C9 and CYP2C19, indicating that its respective IC50 values were 20.68, 29.20 and 18.78 μM (Table 2). IC50 value of CYP2E1 was a higher value (99.58 μM) compared to others mentioned above. In case of CYP3A4, 6-shogaol enhanced midazolam 1-hydroxylation. Because IC50 values are quite different depending on the used substrate concentration as well as enzyme concentration, absolute values (Ki) for expressing inhibitory effect should be estimated. Construction of Dixon plot was performed with appropriate range of substrate concentrations and multiple 6-shogaol concentrations showing inhibitory action on each CYP isoenzyme for estimation of Ki values. Also, an inhibition data set was simulated with three different inhibition models to elucidate the inhibition type of 6-shogaol on each CYP isoenzyme (competitive, noncompetitive, uncompetitive). Representative Dixon and Lineweaver-Burk plot for inhibition of CYP2C19 were shown in Fig. 2. For CYP2C19, inhibition data set was analyzed by simulating with inhibition model, which suggested that a noncompetitive inhibition model was fitted well with estimated Ki value of 19.26 μM. Inhibition of CYP2C9 catalyzed tolbutamide 4-methylhydroxylation by 6-shogaol fit well to noncompetitive inhibition model (Fig. 3). The Ki value obtained from noncompetitive inhibition equation was 29.02 μM. As shown in Fig. 4, 6-shogaol inhibited CYP1A2 catalyzed phenacetin O-deethylation competitively with an estimated Ki value of 6.33 μM.
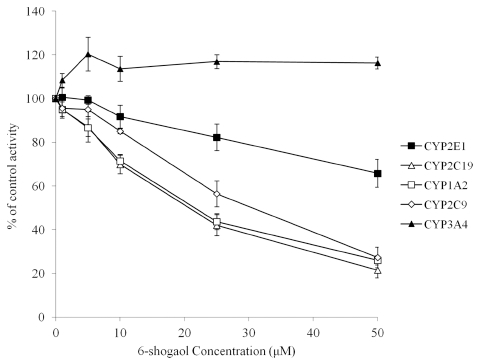
Inhibition of each CYP isoenzymes by 6-shogaol at 0 to 50 μM in human liver microsome.Each point indicates the average of triplicates.
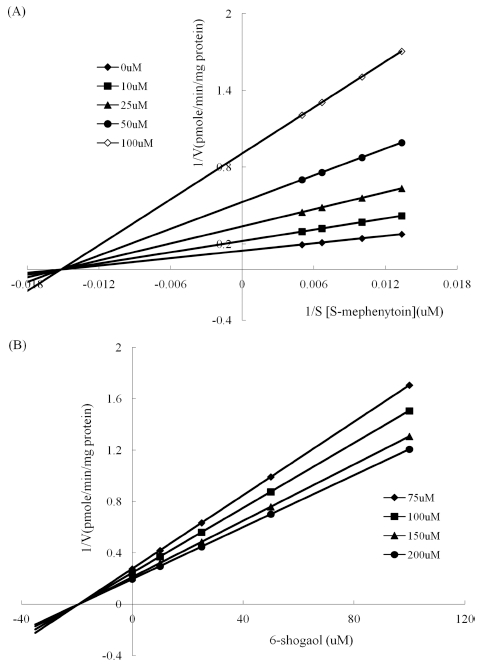
Inhibitory effect of 6-shogaol on CYP2C19 catalyzed S-mephenytoin 4’-hydroxylation.(A); Lineweaver-Burk plot of inhibition of CYP2C19 catalyzed S-mephenytoin 4’-hydroxylation by 6- shogaol. HLM and S-mephenytoin ranging from 75 to 200 μM were incubated with NADPH regenerating system in the absence (◆) or the presence of 10 μM (■), 25 μM (▲), 50 μM (●), 100 μM (◇) of 6- shogaol. (B); Dixon plot of inhibition of CYP2C19 catalyzed S-mephenytoin 4’-hydroxylation by 6- shogaol. The concentrations of S-mephenytoin were 75 μM (◆), 100 μM (■), 150 μM (▲), 200 μM (●). Each point was obtained with the average value of triplicates.
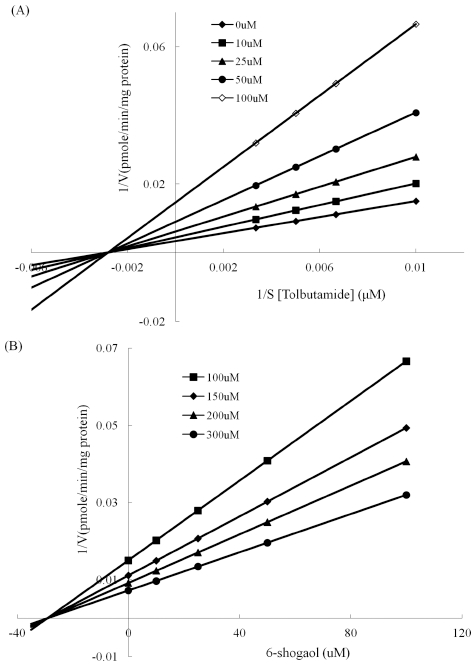
Inhibitory effect of 6-shogaol on CYP2C9 catalyzed tolbutamide 4-methylhydroxylation.(A); Lineweaver-Burk plot of inhibition of CYP2C9 catalyzed tolbutamide 4-methylhydroxylation by 6-shogaol. HLM and tolbutamide ranging from 100 to 300 μM were incubated with NADPH regenerating system in the absence (◆) or the presence of 10 μM (■), 25 μM (▲), 50 μM (●), 100 μM (◇) of 6-shogaol. (B); Dixon plot of inhibition of CYP2C9 catalyzed tolbutamide 4- methylhydroxylation by 6-shogaol. The concentrations of tolbutamide were 100 μM (■), 150 μM (◆), 200 μM (▲), 300 μM (●). Each point was obtained with the average value of triplicates.
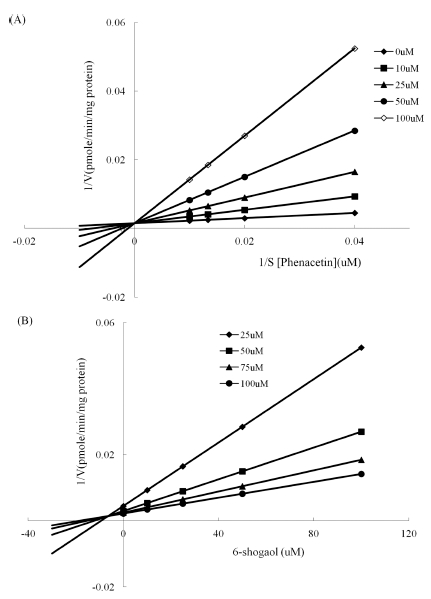
Inhibitory effect of 6-shogaol on CYP1A2 catalyzed phenacetin O-deethylation.(A); Lineweaver-Burk plot of inhibition of CYP1A2 catalyzed phenacetin Odeethylation by 6-shogaol. HLM and phenacetin ranging from 25 to 100 μM were incubated with NADPH regenerating system in the absence (◆) or the presence of 10 μM (■), 25 μM (▲), 50 μM (●), 100 μM (◇) of 6-shogaol. (B); Dixon plot of inhibition of CYP1A2 catalyzed phenacetin Odeethylation by 6-shogaol. The concentrations of tolbutamide were 25 μM (◆), 50 μM (■), 75 μM (▲), 100 μM (●). Each point was obtained with the average value of triplicates.
DISCUSSION
Ginger, the rhizome of Zingiber officinale Roscoe, has been extensively consumed in many countries as a spice in food preparation. In addition to its role of dietary condiment, a function of ginger as a natural herbal medicine has received considerable attentions. Ginger has shown a variety of pharmacological effects like antioxidant, anti-inflammatory, cancer preventive and antineoplastic activity, most of which are attributable to its main pungent constituents, gingerol and shogaol. Like 6-gingerol, 6-shogaol have been revealed to possess remarkable effects such as anti-apoptotic, antioxidant, anti-inflammatory and antiproliferative potencies. Ginger, especially primary constituents of gingerol and shogaol-medicine interactions need to be investigated due to widespread use of ginger in daily life. In a previous study by Joo and Lim (2011), it was reported that 6-gingerol has inhibitory effects on human CYP isoenzymes (Fig. 1, Table 2). The effects of 6-shogaol, a major component of dried ginger, on human metabolic enzymes has not been examined up to date. To elucidate the ginger components-medicine interaction, investigation concerning the effects of 6-shogaol on the CYP isoenzymes with pooled human liver microsome was performed. In this study, it was indicated that among CYP isoenzymes tested, CYP2C19 were proven to be substantially inhibited by 6-shogaol with a noncompetitive inhibition mode (Ki value of 19.26 μM, Fig. 2). Joo and Lim (2011) reported that 6-gingerol inhibited CYP2C19 catalyzed Smephenytoin 4'-hydroxylation in a noncompetitive manner with Ki value of 13 μM. On the basis of these results, it could be concluded that 6-shogaol and 6-gingerol showed the same inhibitory mode on human CYP2C19 with similar Ki values and ginger extract with 6-shogaol and 6- gingerol might have the same effects on CYP2C19. 6- shogaol also exhibited a strong competitive inhibition of CYP1A2 catalyzed phenacetin O-deethylation (Ki value of 6.33 μM, Fig. 3). Joo and Lim (2011) showed that 6- gingerol inhibited CYP1A2 catalyzed phenacetin Odeethylation in a competitive mode with Ki value of 29 μM. In case of CYP1A2, 6-shogaol exhibited much a stronger inhibitory effect than 6-gingerol. In spite of difference in Ki values between two compounds, 6-shogaol and 6-gingerol indicated the same competitive inhibition manner on human CYP1A2 as well. In the case of CYP2C9 catalyzed tolbutamide 4-methylhydroxylation, 6- shogaol had an inhibitory effects in a noncompetitive manner with Ki value of 29.02 μM. In contrast, it was reported that there was no inhibitory activity of entire range of 6-gingerol concentration (0 - 500 μM) on human CYP2C9 by Joo and Lim (2011). The effects of 6- shogaol and 6-gingerol on human CYP2C9 is not consistent compared to those on CYP2C19 and CYP1A2. CYP3A4 is the most abundant CYP isoenzyme in human liver, which is mainly responsible for metabolizing approximately 50% of therapeutic agents. This CYP isoenzyme displays allosteric behavior-binding an effector compound at the site other than the active site of enzyme. Allosteric regulation can be homotropic cooperativity, where both substrate and effector are the same chemical species, or heterotropic cooperativity, where substate and effector are different chemical materials (Tang and Stearns, 2001). Depending on the combination of substrate and effector, CYP3A4 isoenzyme can display either positive cooperativity or negative cooperativity. Previous research examined cooperative binding of midazolam with α-naphthoflavone and testosterone, indicating that the formation of 1- hydroxymidazolam was promoted by α-naphthoflavone (Cameron et al., 2005). Another study investigated effects of spice constituents on CYP3A4 mediated midazolam metabolism in pooled human liver microsome, of which results showed that 6-gingerol, d-limonene, and citral among spice constituents enhanced midazolam 1- hydroxylation (Zhang and Lim, 2008). The result of incubation of pooled human liver microsome with 6- shogaol for CYP3A4 mediated midazolam 1-hydroxylation exhibited enhancement of metabolite by 120%. A hypothesis could be suggested that this might be due to positive heterotropic cooperativity, which indicates that binding of 6-shogaol as an effector at the regulatory site enhances the attraction between active site and substrate. On the basis of these results, it should be considered that 6-shogaol and traditional medicine including ginger main component, 6-shogaol, might cause a potent herbal medicine interaction with drugs which are metabolized through CYP2C9, 2C19 and 1A2. In summary, we have shown the in vitro inhibition effect of 6-shogaol, a herbal oriented compound with various pharmacological properties, on human CYP isoenzymes, indicating that CYP2C9 and CYP2C19 isoenzymes were inhibited noncompetitively and CYP1A2 was inhibited competitively. In the case of CYP3A4, 6-shogaol showed enhancement of metabolite. Predicted with this in vitro study, usage of 6-shogaol in vivo might give rise to the interactions with conventional drugs, beverages, and environmental factors related to CYP isoenzymes inhibited by 6-shogaol. To find out in vivo interactions in detail, further research on the clinical trials of 6-shogaol in humans would be needed to proceed.
ACKNOWLEDGEMENTS
This study has been conducted by the Research Grant of Gwangju Health University in 2014(PJ3014028).
REFERENCES
-
Baliga, MS, Haniadka, R, Pereira, MM, D'Souza, JJ, Pallaty, PL, Bhat, HP, Popuri, S, Update on the chemopreventive effects of ginger and its phytochemicals, Critical Reviews in Food Science and Nutrition, (2011), 51, p499-523.
[https://doi.org/10.1080/10408391003698669]

- Cameron, MD, Wen, B, Allen, KE, Roberts, AG, Schuman, JT, Campbell, AP, Kunze, KL, Nelson, SD, Cooperative binding of midazolam with testosterone and α- naphthoflavone within the CYP3A4 active site: A NMR T1 paramagnetic relaxation study, Biochemistry, (2005), 44, p14143-14151.
-
Court, MH, Von Moltke, LL, Shader, RI, Greenblatt, DJ, Biotransformation of chlorzoxazone by hepatic microsomes from humans and ten other mammalian species, Biopharmaceutics and Drug Disposition, (1997), 18, p213-226.
[https://doi.org/10.1002/(sici)1099-081x(199704)18:3<213::aid-bdd15>3.0.co;2-0]

-
Dugasani, S, Pichika, MR, Nadarajah, VD, Balijepalli, MK, Tandra, S, Korlakunta, JN, Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]- gingerol, and [6]-shogaol, Journal of Ethnopharmacology, (2010), 127, p515-520.
[https://doi.org/10.1016/j.jep.2009.10.004]

-
Ernst, E, Pittler, MH, Efficacy of ginger for nausea and vomiting: A systematic review of randomized clinical trials, British Journal of Anaesthesia, (2000), 84, p367-371.
[https://doi.org/10.1093/oxfordjournals.bja.a013442]

- Izzo, AA, Ernst, E, Interactions between herbal medicines and prescribed drugs: An updated systematic review, Drugs, (2009), 69, p1777-1798.
- Joo, SY, Lim, YC, Inhibitory effects of 6-gingerol on cytochrome P450 in human liver microsomes, The Journal of Korean Society for Clinical Pharmacology and Therapeutics, (2011), 19, p52-58.
-
Kim, JS, Lee, SI, Park, HW, Yang, JH, Shin, TY, Kim, YC, Baek, NI, Kim, SH, Choi, SU, Kwon, BM, Leem, KH, Jung, MY, Kim, DK, Cytotoxic components from the dried rhizomes ofZingiber officinaleRoscoe, Archives of Pharmacal Research, (2008), 31, p415-418.
[https://doi.org/10.1007/s12272-001-1172-y]

-
Pelkonen, O, Breimer, DD, Peter, GW, Luc, PB, Role of environmental factors in the pharmacokinetics of drugs: Considerations with respect to animal models, P-450 enzymes, and probe drugs, Handbook of Experimental Pharmacology, (1994), Springer, New York. NY, USA, p289-332.
[https://doi.org/10.1007/978-3-642-78680-8_10]

- Relling, MV, Aoyama, T, Gonzalez, FJ, Meyer, UA, Tolbutamide and mephenytoin hydroxylation by human cytochrome P450s in the CYP2C subfamily, Journal of Pharmacology and Experimental Therapeutics, (1990), 252, p442-447.
-
Rhode, J, Fogoros, S, Zick, S, Wahl, H, Griffith, KA, Huang, J, Liu, JR, Ginger inhibits cell growth and modulates angiogenic factors in ovarian cancer cells, BMC Complementary and Alternative Medicine, (2007), 7, -44.
[https://doi.org/10.1186/1472-6882-7-44]

-
Shim, S, Kim, S, Choi, DS, Kwon, YB, Kwon, J, Antiinflammatory effects of [6]-shogaol: Potential roles of HDAC inhibition and HSP70 induction, Food and Chemical Toxicology, (2011), 49, p2734-2740.
[https://doi.org/10.1016/j.fct.2011.08.012]

-
Shim, S, Kim, S, Kwon, YB, Kwon, J, Protection by [6]-shogaol against lipopolysaccharide-induced toxicity in murine astrocytes is related to production of brain-derived neurotrophic factor, Food and Chemical Toxicology, (2012), 50, p597-602.
[https://doi.org/10.1016/j.fct.2011.11.042]

-
Shukla, Y, Singh, M, Cancer preventive properties of ginger: A brief review, Food and Chemical Toxicology, (2007), 45, p683-690.
[https://doi.org/10.1016/j.fct.2006.11.002]

-
Tang, W, Stearns, RA, Heterotropic cooperativity of cytochrome P450 3A4 and potential drug-drug interactions, Current Drug Metabolism, (2001), 2, p185-198.
[https://doi.org/10.2174/1389200013338658]

- Tassaneeyakul, W, Birkett, DJ, Veronese, ME, Mcmanus, ME, Turkey, RH, Quattrochi, LC, Gelboin, HV, Miners, JO, Specificity of substrate and inhibitor probes for human cytochromes P450 1A1 and 1A2, Journal of Pharmacology and Experimental Therapeutics, (1993), 265, p401-407.
-
Tsai, TH, Tsai, PJ, Ho, SC, Antioxidant and antiinflammatory activities of several commonly used spices, Journal of Food Science, (2005), 70, pC93-C97.
[https://doi.org/10.1111/j.1365-2621.2005.tb09028.x]

-
Wang, YH, Jones, DR, Hall, SD, Differential mechanismbased inhibition of CYP3A4 and CYP3A5 by verapamil, Drug Metabolism and Disposition, (2005), 33, p664-671.
[https://doi.org/10.1124/dmd.104.001834]

-
Wrighton, SA, Stevens, JC, Becker, GW, Vandenbraden, M, Isolation and characterization of human liver cytochrome P450 2C19: Correlation between 2C19 and S-mephenytoin 4'- hydroxylation, Archives of Biochemistry and Biophysics, (1993), 306, p240-245.
[https://doi.org/10.1006/abbi.1993.1506]

-
Yu, Y, Zick, S, Li, X, Zou, P, Wright, B, Sun, D, Examination of the pharmacokinetics of active ingredients of ginger in humans, AAPS Journal, (2011), 13, p417-426.
[https://doi.org/10.1208/s12248-011-9286-5]

- Zhang, W, Lim, LY, Effects of spice constituents on Pglycoproteinmediated transport and CYP3A4-mediated metabolismin vitro, Drug Metabolism and Disposition, (2008), 36, p1283-1290.
